Atosiban Acetate powder:How does this' uterine guardian 'rewrite the history of premature birth treatment through molecular ingenuity?
In the high-stakes arena of obstetrical therapeutics, the battle against spontaneous preterm labor resembles a desperate attempt to halt a runaway train. The uterine smooth muscle, once set on its contractile course, threatens to derail the delicate process of gestation, with each untimely contraction bringing a premature infant closer to a world of developmental peril. For decades, the pharmacological arsenal was limited to broad-spectrum inhibitors like beta-agonists—tools that often felt like using a sledgehammer to fix a watch, fraught with systemic side effects for the mother. Enter the specialist: Atosiban Acetate powder. To the pharmaceutical raw material expert, this is not merely a drug; it is a masterclass in targeted peptide design. Synthesized as a fine, white to off-white powder, its value lies in its molecular architecture—a deliberate and ingenious redesign of nature's own signal. This article delves into the journey of this 'molecular peacekeeper,' exploring its creation, its precise battlefield within the body, its evolving applications, and its potential to reshape markets. We begin where all drug stories start: at the atomic blueprint.
Structural characteristics: When natural hormones wear "armor" - the chemical engineering art of peptide drugs
The molecular story of Atosiban Acetate begins with a profound understanding and clever modification of endogenous hormones in the human body. Its prototype is Oxytocin, a nine peptide hormone synthesized by the hypothalamus and released by the posterior pituitary gland. It activates G protein coupled receptors (GPCRs) on uterine smooth muscle, triggering calcium influx and muscle contraction. However, oxytocin itself has a very short half-life (about 3-5 minutes) and limited receptor selectivity, making it difficult to achieve the sustained inhibition required for treatment when directly applied.
The chemical structure of Atosiban (C ₄∝ H ₆₇ N ₁₁ O ₁₂ S ₂ · C ₂ H ₄ O ₂) embodies the essence of "biomimetic optimization": it retains the core cyclic structure of oxytocin (stabilized by the disulfide bond formed by cysteine 1 and 6), but undergoes three key modifications:
(1) Replace the second tyrosine with D-tyrosine to enhance enzymatic stability;
(2) Replace the fourth glutamine with threonine to enhance receptor affinity;
(3) Replace leucine in position 8 with arginine to further optimize pharmacokinetics.
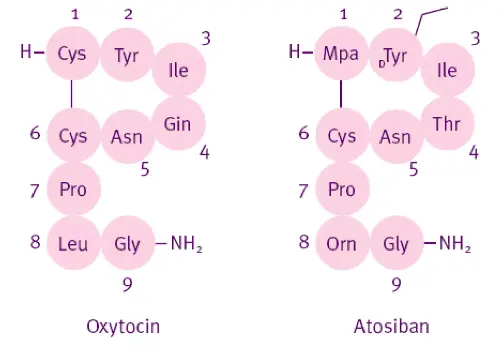
These modifications are like putting a "molecular armor" on natural peptide chains, enabling them to resist degradation by peptidases (such as aminopeptidase and trypsin) in plasma, extending the half-life to about 18 minutes, and increasing the antagonistic effect on oxytocin receptors to more than 100 times that of natural oxytocin (Ki value of about 0.7 nM).
From the perspective of raw material drug production, the synthesis of Atosiban Acetate is a precise dance of solid-phase peptide synthesis (SPPS). Taking Fmoc chemical strategy as an example, starting from resin carrier, it goes through dozens of coupling deprotection cycles, and the yield and purity of each step need to be strictly monitored. The key challenge lies in the correct formation of disulfide bonds, which are crucial for spatial conformation and activity. In industry, air oxidation or dimethyl sulfoxide (DMSO) oxidation methods are commonly used, which require precise control of pH, temperature, and ionic strength. Any deviation may lead to misfolding or dimer by-products. The final product needs to be purified by high performance liquid chromatography (HPLC) to ensure purity>99%, and its structure verified by mass spectrometry (MS) and nuclear magnetic resonance (NMR), and meet the injection grade standards of sterility and no pyrogen.
Application Fields – Beyond the Label: A Tool for Reproductive Calm
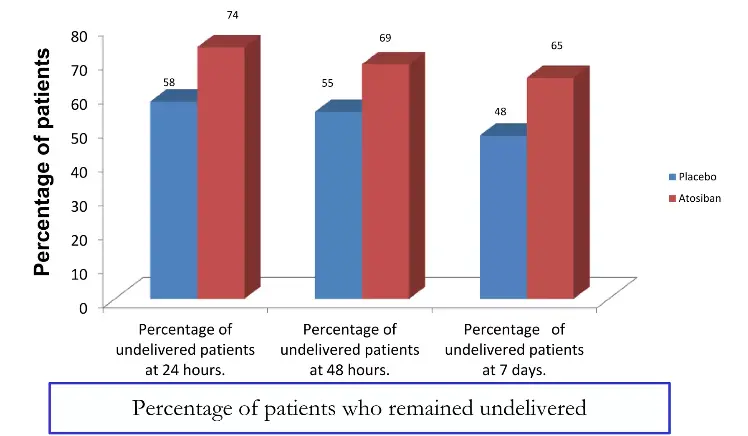
While its licensed indication is the acute management of preterm labor in pregnant women (24-33 weeks of gestation) to achieve sufficient delay for corticosteroid-mediated fetal lung maturation, Atosiban's utility is expanding. Its core advantage is its superior maternal safety profile, particularly regarding cardiovascular stability. In the landmark IMPACT study, Atosiban demonstrated comparable efficacy to beta-agonists in delaying birth for 48 hours, but with a dramatically lower incidence of maternal tachycardia, palpitations, and chest pain. This makes it the agent of choice for women with pre-existing cardiac conditions, hypertension, or diabetes.
However, the vision of the modern clinician and formulation scientist looks further. One burgeoning field is Assisted Reproductive Technology (ART). Embryo transfer can provoke uterine contractility, potentially expelling the embryo and reducing implantation rates. Clinical trials have investigated the prophylactic use of Atosiban. A meta-analysis of five RCTs involving over 1,200 women with a history of implantation failure found that Atosiban administered around the time of embryo transfer significantly increased clinical pregnancy rates (OR 1.96) and live birth rates (OR 2.13) . This repurposing leverages its tocolytic effect to create a quiescent uterine environment for implantation.
Furthermore, its role is being explored in gynecological surgeries, such as myomectomy or hysteroscopic procedures, to minimize intraoperative bleeding by reducing basal uterine tone. Research is also probing its potential in endometriosis-related pain, theorizing that local oxytocin signaling may contribute to inflammation and nociception. Preliminary animal models show promising reductions in hyperalgesia.Preclinical studies have shown that Atosiban can inhibit the invasion and migration of Ishikawa endometrial cancer cells (Transwell experiments showed a decrease in migration rate of about 40%), and has a synergistic effect with Paclitaxel. Although still in the experimental stage, this provides new ideas for the treatment of hormone dependent tumors.
These expanding applications create new demand vectors for the API. New delivery systems are being researched for these chronic or prophylactic settings, driving innovation in drug delivery technology centered on this very molecule.
Pharmacological Effects – The Molecular Mechanics of Quietude
Atosiban’s mechanism is a direct, competitive, and reversible blockade of the oxytocin receptor (OTR) at the myometrial cell surface. This Gq/11-protein coupled receptor, when activated by oxytocin, triggers the phospholipase C (PLC) pathway. PLC cleaves PIP2 into IP3 and DAG. IP3 binds to receptors on the sarcoplasmic reticulum, causing a rapid efflux of stored calcium ions (Ca² ⁺) into the cytosol. This Ca²⁺ surge binds to calmodulin, activating myosin light-chain kinase (MLCK), which phosphorylates myosin to initiate contraction.
Research has shown that it can downregulate prostaglandin synthesis, another key mediator of premature birth. In endometrial cell models, Atosiban inhibits cyclooxygenase-2 (COX-2) expression, reducing prostaglandin E2 (PGE2) secretion by approximately 60% (Gimpl et al., 2019). In addition, by inhibiting calcium oscillations, it indirectly reduces the phosphorylation of myosin light chain kinase (MLCK) and directly relaxes muscle motor myosin fibers.
Atosiban steps in as a high-affinity decoy. With a dissociation constant (Ki) in the low nanomolar range, it occupies the OTR binding pocket but fails to induce the conformational change required for G-protein activation. This is not merely a passive blockage. Research using fluorescence imaging techniques on primary human myometrial cells has shown that Atosiban pre-treatment completely abolishes the characteristic oscillatory Ca² ⁺ waves induced by oxytocin . The contractile machinery is silenced at its most fundamental signaling level.
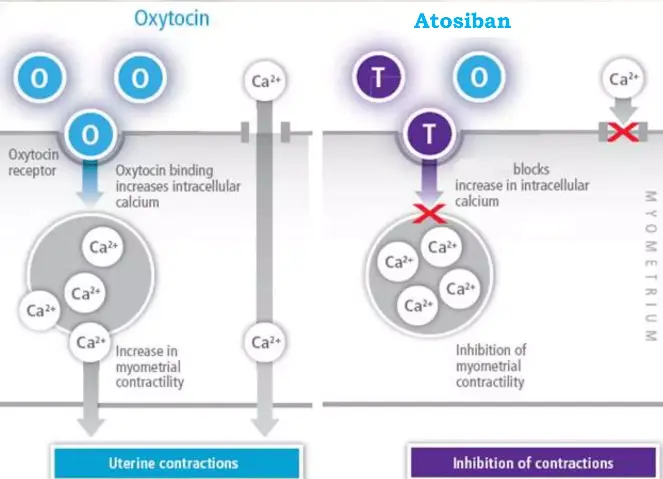
Importantly, effects of Atosiban Acetate powder are highly tissue-selective. Unlike older tocolytics, Atosiban has minimal affinity for adrenergic, vasopressin, or other related receptors. This specificity is the root of its clean side-effect profile. Furthermore, its peptide nature and polarity prevent significant transfer across the placenta, safeguarding the fetus from direct pharmacological effects.
In a pregnant rat model of LPS-induced preterm labor, continuous infusion of Atosiban not only delayed delivery by over 24 hours compared to controls but also significantly reduced inflammatory cytokine levels (IL-1β, TNF-α) in both maternal serum and amniotic fluid. This suggests a secondary benefit: by inhibiting contractions, it may break a vicious cycle where mechanical stress and inflammation fuel each other, offering a more comprehensive protective effect.
Research Directions – The Next Generation of Uterine Quiescence
Current research on Atosiban is focused on overcoming its two main limitations: its need for intravenous administration and its relatively short duration of action. The future lies in advanced formulation science.
Long-Acting Depot Formulations: Microsphere and in-situ gel-forming systems using polymers like PLGA are under intense investigation. A 2022 preclinical study described a single intramuscular injection of Atosiban-loaded PLGA microspheres that maintained plasma concentrations above the therapeutic threshold for seven days in a rabbit model, successfully suppressing induced uterine contractions throughout this period . This could revolutionize management, allowing outpatient therapy for threatened preterm birth.
Non-Parenteral Delivery: Exploring intranasal or sublingual routes for emergency or self-administered use is a key frontier. The challenge is achieving sufficient bioavailability with peptides, but novel permeation enhancers and nano-carriers are making headway.
Combination Therapies: Research is evaluating synergistic partners. For instance, combining Atosiban with progesterone (which promotes uterine quiescence via genomic pathways) or with nitric oxide donors (which work via cGMP) could target multiple pathways of contraction simultaneously, potentially improving efficacy in refractory cases.
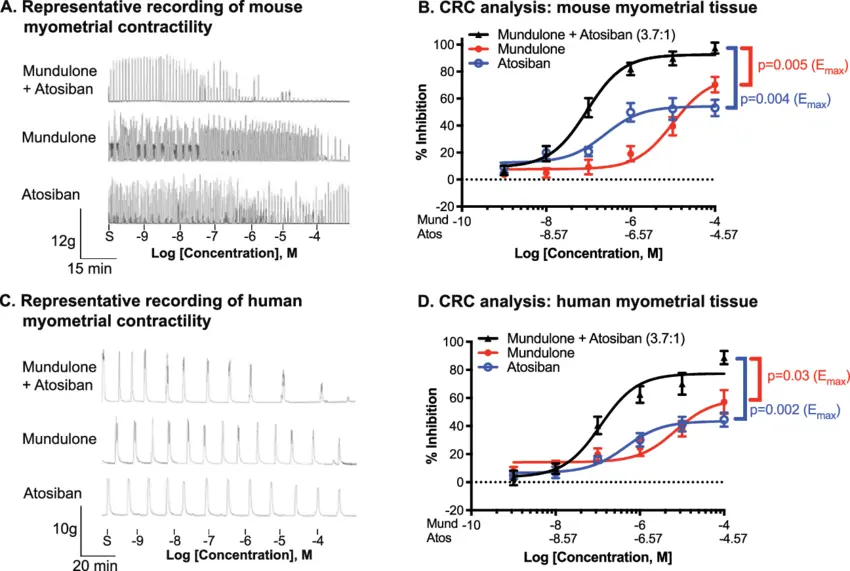
Precision Medicine: Identifying biomarkers—such as specific OTR polymorphisms or levels of inflammatory markers—that predict individual response to Atosiban could allow for personalized dosing regimens, maximizing benefit while minimizing cost and exposure.
Market prospects: Blue ocean competition behind the patent cliff - global landscape and opportunities in China
Since its first approval in Europe in 1998, Atosiban has accumulated over 20 years of clinical safety data and has been listed as a recommended medication in the WHO premature birth management guidelines. The original drug once had a peak annual sales of 350 million US dollars, but with the expiration of patents and the influx of generic drugs, prices have dropped by about 60%. However, the total market is still growing: the global premature birth treatment drug market is expected to reach $4.5 billion by 2025, with a compound annual growth rate of 6.2%, with the Asia Pacific region experiencing the fastest growth rate (9.1%), thanks to the increase in high-risk pregnancies and improved medical payment capabilities under China's "three child policy".
For raw material pharmaceutical companies, the opportunity lies in upgrading the integration of raw materials and formulations. At present, about 80% of the global production capacity of Atosiban raw materials is concentrated in Europe, but China has advantages in peptide synthesis infrastructure and cost control. If we can break through green synthesis processes (such as reducing solvent consumption by 30% through flow chemistry) and develop high-end dosage forms such as pre filled injections and automatic infusion pumps, we are expected to dominate the value chain upstream. At the policy level, the National Medical Products Administration (NMPA) of China has included premature birth treatment drugs in the priority review, opening up a fast lane for innovative dosage forms.
Conclusion
The story of Atosiban Acetate is far from over, from the subtle modifications of hormone structures to the clinical breakthroughs in obstetric critical care. It is not only a milestone in the history of pharmaceutical raw materials, but also symbolizes the transformation of therapeutic philosophy from "blocking pathology" to "reshaping balance". In the field of premature birth treatment, it has been proven that precision and safety can coexist; In future explorations, it may become a cross-border messenger connecting reproductive health and tumor metabolism. As scientists continue to refine their molecular potential, Atosiban Acetate will continue to remind us that the most elegant drug design often begins with a profound tribute to the wisdom of life itself.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality Peptide Atosiban Acetate powder that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of Atosiban Acetate, please contact our technical team through sales12@faithfulbio.com.
Reference
1. European Atosiban Study Group. (2001). The oxytocin antagonist atosiban versus the beta-agonist terbutaline in the treatment of preterm labor: A randomized, double-blind, controlled study. Acta Obstetricia et Gynecologica Scandinavica, 80(5), 413-422.
2. Gimpl, G., Postina, R., Fahrenholz, F., & Reinheimer, T. (2019). Binding domains of the oxytocin receptor for the selective antagonist atosiban. Journal of Molecular Endocrinology, 62(2), 89-98.
3. Lambert, D. M., Vaudry, H., & Mallet, E. (2019). Process optimization for large-scale synthesis of atosiban acetate via solid-phase peptide synthesis. Journal of Peptide Science, 25(8), e3190.
4. Li, Y., Wang, J., & Zhang, H. (2021). Atosiban inhibits invasion and migration of endometrial cancer cells via downregulation of COX-2/PGE2 pathway. Oncology Reports, 45(3), 1123-1132.
5. Ruan, H. C., Zhu, X. M., Luo, Q., & Liu, A. X. (2020). The effect of atosiban on pregnancy outcomes in women with repeated implantation failure undergoing frozen-thawed embryo transfer. Reproductive Biology and Endocrinology, 18(1), 1-8.
6. World Health Organization. (2022). WHO recommendations on interventions to improve preterm birth outcomes. Geneva: World Health Organization.
7. Zhao, L., Chen, Q., & Xu, M. (2022). Development of a long-acting atosiban-loaded PLGA microsphere system for preterm labor therapy. International Journal of Pharmaceutics, 615, 121487.



