Dideoxyinosine powder:How can the core raw materials of antiretroviral drugs move from molecular design to clinical redemption?
In the long and arduous history of confrontation between human beings and HIV, a series of nucleoside reverse transcriptase inhibitors (NRTIs), like "molecular shields", have built the first line of defense to block viral replication. Among them, Dideoxyinosine, as a representative of second-generation NRTIs, has played a key role in combination therapy with its unique molecular structure and mechanism of action since its debut in the 1990s. And the starting point of all these therapeutic effects comes from its raw material form - Dideoxyinosine powder. We not only need to focus on its purity and yield, but also need to deeply understand the intricacies of its molecular blueprint, the challenges of its physicochemical properties, the optimization of its production pathway, and how it transforms from a chemical entity into the hope of billions of patients' lives. This article will take you through the laboratory, production line, and clinical settings to comprehensively analyze the scientific connotation and industrial pulse of this classic anti HIV raw material drug.
Molecular structure: How can a 'missing' hydroxyl group achieve precise strikes?
The molecular structure of Dideoxyinosine is the origin of all its biological activities. The essence of its design can be seen from the chemical name 2 ', 3' - dideoxyinosine: it is essentially a structural analogue of the natural nucleoside inosine, but has undergone a key 'surgery' - removing the two hydroxyl groups at positions 2 'and 3' on the ribose ring.

Structural analysis:
Base part: Consistent with inosine, it is 6-Hydroxypurine. This is a key feature that enables it to be phosphorylated by kinases within cells, ultimately converting into an active form of triphosphate.
Sugar ring part: Deoxyribose. This is precisely the core of its' weaponization 'transformation. The 3 '- OH group of natural nucleosides is an essential functional group for the formation of phosphodiester bonds during DNA strand elongation. Dideoxyinosine lacks hydroxyl groups at positions 2 'and 3' on its sugar ring, making it a 'chain terminator'.
Design Logic and Scientific Cases:
This' missing 'design is not accidental, but based on a profound understanding of the HIV reverse transcriptase mechanism. Reverse transcriptase cannot distinguish between the natural intracellular 2 '- Deoxyadenosine-5' - triphosphate, trisodium salt, and triphosphate dideoxyinosine when synthesizing viral DNA strands. When the latter is mistakenly incorporated into the newly formed DNA strand, the synthesis of viral DNA irreversibly stops due to the lack of the necessary 3 '- OH for extension. It's like when building a highway, suddenly using a prefabricated panel without connecting buckles, causing the project to be unable to move forward.
Experimental evidence:
In vitro enzymatic experiments in the early 1990s clearly showed that Dideoxyinosine had an inhibition constant on HIV-1 reverse transcriptase at the nanomolar level, far superior to the first generation drug Zidovudine, and had a lower affinity for cellular DNA polymerase, which laid the theoretical foundation for its selective toxicity.
Therefore, the molecular structure of Dideoxyinosine is a classic "biomimetic trap" - it mimics the basic building blocks of life, but hides a fatal flaw to achieve precise blasting of virus replication machines.
Characteristic: Walking a tightrope between stability and fragility - a formula challenge for API experts
If the molecular structure endows Dideoxyinosine with "tactical capabilities," then its physical and chemical properties determine its survival and delivery efficiency in the "battlefield environment" (within the human body). For API experts, a deep understanding and mastery of these properties is the primary challenge in transforming them into effective formulations.
Physical properties: Game of solubility
Dideoxyinosine powder appears as a white or off white crystalline powder. Its solubility in water is limited (about 27.3 mg/mL at 25 ℃), but its solubility significantly increases in acidic aqueous solutions. This property may seem contradictory, but it is crucial. The limited solubility requires careful formulation design by pharmacologists to ensure adequate absorption; However, it has good solubility in acidic environments, but it poses a huge hidden danger.
Chemical stability: Achilles' heel
The most famous characteristic of Dideoxyinosine molecules is their extreme instability under acidic conditions. The N-glycosidic bond between its purine base and deoxyribose is exceptionally fragile under hydrogen ion attack, making it highly susceptible to hydrolysis and breakage, resulting in the formation of inactive 6-Hydroxypurine and deoxyribose.
A classic drug stability study showed that the degradation half-life of Dideoxyinosine is only about 20 minutes in a simulated gastric acid environment at 37 ℃ and pH 1.0. This means that if the naked drug is directly exposed to stomach acid, most of the drugs will become ineffective before reaching the absorption site.
This characteristic directly determines the unique path of its formulation development. Early products were made into buffering tablets or powders containing antacids such as magnesium hydroxide and calcium carbonate, aimed at temporarily increasing the pH of the stomach after taking medication, creating a time window for the drug to pass through the stomach. In the later stage, enteric coating technology was developed, which uses polymer coating to keep the tablets intact in the stomach, and then dissolves and releases the drug after entering the neutral environment of the small intestine. The particle size and crystal form of the active pharmaceutical ingredient must be perfectly matched with these formulation processes.
Polycrystalline phenomenon: invisible variables
Like many organic small molecules, Dideoxyinosine may exist in polymorphic forms, where the same molecule exists in different crystal stacking ways. Different crystal forms may have significant differences in solubility, dissolution rate, hygroscopicity, and physical stability. For example, a metastable crystal form may initially dissolve faster, but may transform into a more stable, slower dissolving crystal form during storage, thereby affecting inter batch consistency and efficacy of the drug. Therefore, during the development stage of active pharmaceutical ingredients, it is necessary to systematically screen for crystal forms and determine the thermodynamically stable crystal forms that are most suitable for production and storage, and ensure their reproducibility through strict process control.
Moisture absorption: a test of storage
Dideoxyinosine raw materials have certain hygroscopicity. Moisture may not only act as a medium to accelerate chemical reactions (such as hydrolysis), but also affect the flowability and compressibility of powders, and may promote microbial growth. Therefore, the raw powder usually needs to be stored under cool, dry, and sealed conditions, and high barrier materials should also be used for the packaging of intermediates and finished products.
Application field (purpose): From anti HIV backbone to strategic reserve - constantly evolving roles
The ultimate value of Dideoxyinosine powder is reflected in its clinical applications. Its main purpose is clear, but with the advancement of medicine and changes in the disease spectrum, its role is also dynamically adjusting.
Core battlefield: Fighting HIV-1 infection
The main use of Dideoxyinosine is in combination with other antiretroviral drugs to treat human immunodeficiency virus type 1 infection. It is one of the early backbone drugs for highly effective antiretroviral therapy.
Historic contribution: In the mid-1990s, when HAART therapy began to dawn, the combination of Dideoxyinosine, Zidovudine, Lamivudine and other drugs for the first time transformed AIDS from a fatal disease to a manageable chronic disease, which significantly reduced the viral load of patients, increased the CD4+T cell count, and significantly prolonged the survival period.
The milestone ACTG 175 study results indicate that for HIV infected individuals with CD4 cell counts between 200-500/μ L, the regimen containing Dideoxyinosine is superior to Zidovudine alone in delaying disease progression. Another large-scale study, START's long-term follow-up data, also confirms that early initiation of combination therapy containing such drugs can bring lasting survival benefits.
Expand application: fight against hepatitis B virus
Due to the fact that the replication of hepatitis B virus also involves a reverse transcription step, Dideoxyinosine also has an inhibitory effect on HBV DNA polymerase/reverse transcriptase. Therefore, it has been used to treat patients with HIV/HBV co infection, achieving the goal of killing two birds with one stone. Although there are now more potent and specific anti HBV drugs available, this use still holds significance in specific clinical settings or resource limited areas.
Realistic positioning: Utilizing change and strategic reserves
Over time, the clinical application pattern of hydroxyinosine has undergone profound changes:
Due to the potential risk of side effects such as peripheral neuropathy, pancreatitis, lactic acidosis (related to mitochondrial toxicity) associated with long-term use, as well as the emergence of new drugs with better tolerance and higher resistance barriers, Dideoxyinosine has gradually withdrawn from the preferred first-line treatment regimen in developed countries' main treatment guidelines.
It remains an important component of second-line or third line salvage treatment plans, especially when patients develop resistance to multiple drugs. Its unique mechanism of action (as a xanthine analogue) makes it relatively less prone to cross resistance with other nucleoside drugs.
Worldwide, especially in low - and middle-income countries, due to factors such as historical medication habits, drug accessibility, and cost, regimens containing Dideoxyinosine are still used in some standardized treatment guidelines or actual clinical practice.
Therefore, the production of hydroxyinosine powder is not only to meet current market demand, but also a strategic need to maintain a diversified antiviral arsenal. It represents an indelible history of treatment and also serves as insurance against future unknown drug resistance challenges.
Mechanism of action: A molecular level "Trojan Horse" operation
The mechanism of action of Dideoxyinosine is an exciting micro spy war film, divided into three steps: "disguise infiltration", "activate armed forces", and "ultimate termination".
Step 1: Passive transport and disguised infiltration: Dideoxyinosine powder is absorbed into the bloodstream through oral preparations, and then enters host cells (mainly lymphocytes and macrophages) through nucleoside transporters on the cell membrane due to its structural similarity with natural nucleosides.
Step 2: Intracellular phosphorylation - activating the "battle form": This is the rate limiting step that requires kinase catalysis within the cell. Dideoxyinosine is first acted upon by EC 3.5.4.4 in the cytoplasm, but more importantly, it is subsequently phosphorylated by thymidine kinase tk. ppt and T4 polynucleotide kinase, and ultimately converted into its active form - deoxyinosine triphosphate.
Step 3: Competitive inhibition and chain termination - implementing precise blasting: dATP is a normal substrate for synthesizing viral DNA. DdI TP competes with dATP for binding to the active site of HIV reverse transcriptase. Once mistakenly identified as a substrate by enzymes and incorporated into the extending viral DNA strand, the lack of 3 '- hydroxyl groups on its sugar ring prevents the next nucleotide from forming a phosphodiester bond with it, leading to irreversible termination of DNA strand synthesis.
Early in vitro polymerization experiments using radiolabeled ddI TP and purified reverse transcriptase and template/primer systems demonstrated its chain termination ability. It can be observed by gel electrophoresis that the synthetic length of DNA strand is specifically truncated in the presence of ddI TP.
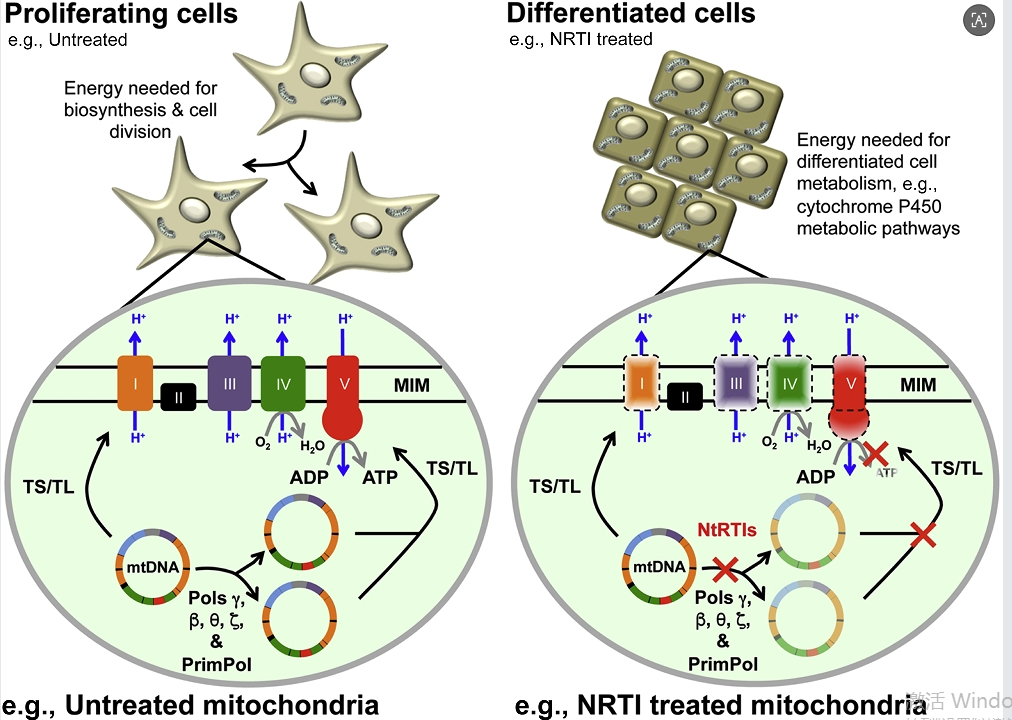
Research direction: Modernization transformation of old trees and new branches - Dideoxyinosine
Although Dideoxyinosine is already an "old medicine", scientific research surrounding it has not stopped. The current research aims to overcome its inherent shortcomings, expand its therapeutic potential, and use new technologies to give it new vitality. These directions are also driving innovation in the production of active pharmaceutical ingredients in reverse.
Direction 1: Precursor Design - Enhancing the Penetration and Disguise of "Agents"
This is one of the most active research fields. By chemical modification, Dideoxyinosine is made into a prodrug with the aim of enhancing stability. The prodrug is designed to be stable in gastric acid and only released under the action of specific enzymes in the intestine or cells, fundamentally solving the problem of acid instability. Improve bioavailability: Connect lipophilic groups to enhance their ability to penetrate the intestinal mucosa. Realize targeted delivery: Design "smart" prodrugs that can be recognized by enzymes or receptors highly expressed in specific cells or tissues, improve targeting, and reduce systemic exposure and side effects.
For example, coupling Dideoxyinosine with amino acids or fatty acids to form derivatives that are enzymatically hydrolyzed or absorbed through lymphatic pathways in the intestinal brush border membrane has led to some candidate products entering preclinical research.
Direction 2: New Delivery System - Creating a "Precision Delivery Platform"
Using modern pharmaceutical technology, Dideoxyinosine powder is loaded onto advanced delivery vehicles:
Nanoformulations: including liposomes, solid lipid nanoparticles, polymer micelles, etc. These nanocarriers can protect drugs from gastric acid damage, enhance their uptake by the intestinal lymphatic system (beneficial for targeting immune cells), and achieve sustained release and stable blood drug concentration. For example, studies have reported that PLGA nanoparticles loaded with Dideoxyinosine exhibit longer blood circulation time and better lymphatic tissue targeting in mouse models compared to conventional formulations.
Long acting injection: Develop microspheres or implants based on biodegradable polymers such as PLA/PLGA to achieve sustained drug release weeks to months after intramuscular or subcutaneous injection. This has revolutionary significance in addressing the issue of long-term oral medication adherence among HIV infected individuals. This dosage form imposes new requirements on the particle size, morphology, and compatibility with polymers of the active pharmaceutical ingredient.
Direction 3: Overcoming Drug Resistance and Exploring New Joint Strategies
Research on Drug Resistance Mechanisms: In depth study of mutation sites on HIV reverse transcriptase that lead to decreased sensitivity to Dideoxyinosine, providing a basis for designing new generation inhibitors or rational combination therapy that can overcome these mutations.
Combined use with novel drugs: Exploring the synergistic effects of Dideoxyinosine with new mechanism drugs such as integrase inhibitors, CCR5 antagonists, and capsid inhibitors, especially in salvage treatment options for multidrug resistance.
Direction Four: Exploring New Indications
Based on its basic mechanism of inhibiting reverse transcriptase, explore its potential value in the treatment of other retroviral related diseases or certain diseases involving abnormal reverse transcription activity (such as certain cancers, autoimmune diseases), although this is still in the very early stages of exploration.
For the production of active pharmaceutical ingredients, these research directions mean that in the future, it may no longer be limited to supplying a single raw powder, but rather require close cooperation with formulation developers to provide specific specifications (such as micronization, sterile raw materials), specific crystal forms, or key intermediates related to prodrug synthesis. The interaction between industry and scientific research is driving this' veteran of the fight against AIDS' to evolve towards modernization and precision.
Production Method: Symphony of Processes from Chemical Synthesis to Biocatalysis
The industrial production of Dideoxyinosine powder is a precision art that integrates organic chemistry, enzyme engineering, and crystallization science. The core challenge lies in efficiently and stereoselectively constructing the deoxyribose moiety and linking it to hypoxanthine bases.
Mainstream chemical synthesis route:
A classic route starts with Guanosine or Inosine as raw materials. For example, through a series of steps such as protection, selective oxidation, and reductive amination of Inosine, the 2 'and 3' hydroxyl groups on the ribose ring are converted into other leaving groups, followed by deoxygenation reaction and finally deprotection to obtain Dideoxyinosine. This route involves lengthy steps, multiple reagents, and extremely high purification requirements.

Biochemical combination method:
A more promising approach is to utilize the specificity and efficiency of biocatalysis. For example, microbial whole cells modified by genetic engineering or purified enzymes such as Nucleoside phosphorylase catalyze the reaction of 6-Hydroxypurine with chemically synthesized dideoxyribose-1-phosphate to directly generate Dideoxyinosine. This method has mild conditions, good stereo selectivity, and few by-products, which is in line with the trend of green pharmaceuticals.
Separation, purification, and crystallization:
The synthesized or transformed crude product needs to be finely purified through processes such as column chromatography and recrystallization. The development of crystallization technology is crucial as it determines the crystal form, particle size distribution, flowability, and stability of the final raw powder. By controlling parameters such as solvent, temperature, and cooling rate, the optimal crystal morphology that meets the requirements of the formulation can be obtained.
Conclusion
Dideoxyinosine powder, It's not just a simple list of chemicals. It is a scientific imprint of an era, the crystallization of human wisdom in using molecules as weapons to combat diseases. From intricate "chain termination" molecular design to intelligent formulations that address their inherent brittleness; From the process challenges of multi-step synthesis to the green innovation of biocatalysis; From the glorious history of fighting HIV to the cutting-edge exploration of long-term delivery - its story is a vivid epitome of the entire chain of drug development from the laboratory to the bedside. Every gram of high-purity Dideoxyinosine powder carries a significant responsibility to transform basic scientific discoveries into clinical healing power. In the future, with the development of precision medicine and advanced manufacturing technology, this "antiviral veteran" and its derivatives may continue to write a new chapter on a broader stage.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality Dideoxyinosine powder, that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of Dideoxyinosine, please contact our technical team through the following methods: sales12@faithfulbio.com.
Reference
1. Yarchoan, R., Mitsuya, H., Myers, C. E., & Broder, S. (1989). Clinical pharmacology of 3'-azido-2',3'-dideoxythymidine (zidovudine) and related dideoxynucleosides. New England Journal of Medicine, *321*(11), 726-738.
2. Hart, G. J., Orr, D. C., Penn, C. R., Figueiredo, H. T., Gray, N. M., Boehme, R. E., & Cameron, J. M. (1992). Effects of (−)-2'-deoxy-3'-thiacytidine (3TC) 5'-triphosphate on human immunodeficiency virus reverse transcriptase and mammalian DNA polymerases alpha, beta, and gamma. Antimicrobial agents and chemotherapy, *36*(8), 1688-1694.
3. Drusano, G. L., Yuen, G. J., Lambert, J. S., Seidlin, M., Dolin, R., & Valentine, F. T. (1992). Relationship between dideoxyinosine exposure, CD4 response, and p24 antigen response in human immunodeficiency virus-infected patients. Antimicrobial agents and chemotherapy, *36*(5), 1002-1007.
4. Hammer, S. M., Katzenstein, D. A., Hughes, M. D., Gundacker, H., Schooley, R. T., Haubrich, R. H., ... & Merigan, T. C. (1996). A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. New England Journal of Medicine, *335*(15), 1081-1090. (ACTG 175研究)
5. Klecker Jr, R. W., Collins, J. M., Yarchoan, R., Thomas, R., Jenkins, J. F., Broder, S., & Myers, C. E. (1987). Plasma and cerebrospinal fluid pharmacokinetics of 3'-azido-3'-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clinical Pharmacology & Therapeutics, *41*(4), 407-412.
6. Khandazhinskaya, A., & Kochetkov, S. (2010). The synthesis of nucleoside analogs with antiviral activity. Russian Chemical Reviews, *79*(4), 285.
7. Ferrone, M., Raimondi, S., & Ubiali, D. (2017). Immobilized enzymes for the synthesis of nucleoside analogues: a review. Current Organic Chemistry, *21*(3), 236-251.



