Peptide powder Retatrutide: How can this "triple agonist" powder reshape the treatment pattern of metabolic diseases?
In the rapidly evolving field of metabolic disease treatment, the success of GLP-1 receptor agonists such as semaglutide seems to have opened the door to a new world for us. However, the pace of science has never stopped, and researchers have already set their sights further ahead. We can't help but ask: Is there a more powerful molecule that can precisely attack multiple pathological links of obesity, diabetes and related complications at the same time? At this moment, the spotlight suddenly illuminated a rising star - Peptide Powder Retatrutide. It is not just an upgraded version of GLP-1 drug, but also a carefully designed trio of masters. Today, let's unveil the mysterious veil of this' metabolic missile '.
Molecular Structure: A Carefully choreographed "Polypeptide Symphony"
When we talk about Peptide Powder Retarutide, the first thing we face is a "solid-state symphony" encoded precisely by amino acids. This is not a simple chemical formula, but a complex artwork that showcases the pinnacle of modern biopharmaceutical technology. What we see is not only the functional label of its GIP-GLP-1-glucagon "triple agonist", but also the precise structural basis for its implementation of this function.
The core skeleton of Retatrutide is a carefully modified single chain peptide of 39 amino acids. It has a homologous lineage with natural GLP-1 (glucagon like peptide-1), but its greatness lies in a series of "ingenious" structural modifications. Firstly, the most striking feature is the huge "lipophilic anchor" composed of two C-20 fatty acid chains connected by a branched chain on its 20th lysine. This design is not accidental, it is the 'stabilizing sea needle' of the entire molecular pharmacokinetic properties. In the body, this massive hydrophobic structure can undergo high-intensity and reversible binding with albumin in the blood. This combination is like a combination of an "invisibility cloak" and a "time capsule": on the one hand, it allows Retatrutide to "hide" in the bloodstream, avoiding rapid filtration and clearance by the kidneys; On the other hand, it creates a huge circulating drug reserve that can continuously and slowly release active free drugs, thereby extending their half-life to an astonishing 100 hours or more.
In addition, to combat the ubiquitous "molecular scissors" in the body - dipeptidyl peptidase-4 (DPP-4), the researchers performed a clever surgery on the second amino acid of the Retarutide sequence, replacing natural alanine with Aminoisobutyric Acid (Aib). Aib is a non natural amino acid whose spatial structure can effectively "deceive" the active center of DPP-4, making it unable to recognize and cleave Retarutide. This tiny 'switch' is like adding a sturdy lock to the core movement of this symphony, ensuring that the drug will not be 'dismembered' in the bloodstream before entering the target, greatly improving its biological stability.
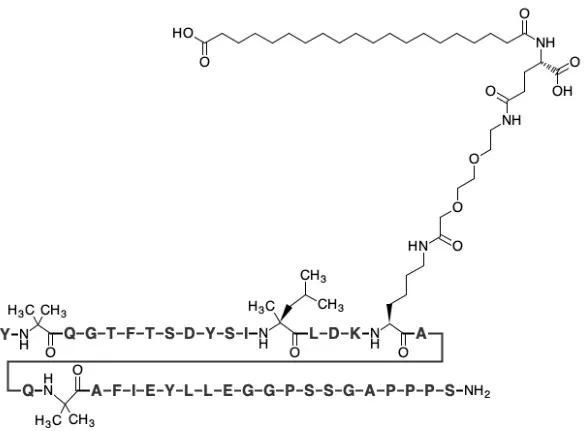
From the perspective of three-dimensional spatial structure, the design of Retatrutide aims to enable it to simultaneously "embrace" three different B-class G protein coupled receptors (GPCRs) with high affinity: GLP-1 receptor, GIP receptor, and glucagon receptor. The C-terminal domain has been optimized to accommodate the binding pocket of GLP-1R and GIPR; And its middle and N-terminal regions are designed to simulate natural glucagon, effectively activating GCGR. The ability of "one stone, three birds" is not simply the concatenation of three independent peptide segments, but rather the selective activation of different receptors through a single, coherent peptide sequence and its conformational flexibility. This requires that the raw materials must maintain extremely high purity and correct spatial folding during the production process, as any incorrect folding or polymerization may affect their efficacy and safety.
Therefore, every gram of our Retatrutide powder embodies the wisdom of structural biology, medicinal chemistry, and process engineering. It is not a static chemical molecule, but a dynamic life tool endowed with a precise mission. Every detail of its structure is designed to play a coordinated, long-lasting, and powerful metabolic regulation movement in the human body.
Usage: A metabolic "all powerful warrior" that surpasses the "weight loss miracle drug"
If Peptide powder Retatrutide is only regarded as a powerful weight loss drug, it undoubtedly underestimates its enormous potential. The use of a raw material is determined by its mechanism of action, and the "triple activation" characteristic of Retatrutide determines that it is a "versatile" strategic weapon targeting a wide spectrum of metabolic diseases.
Firstly, in the field of obesity treatment, it is redefining the 'weight loss ceiling'. In the clinical study codenamed "TRIUMPH", Retatrutide demonstrated unprecedented weight loss effects. At 48 weeks, the 12 mg dose group achieved a weight loss of up to 24.2%, which means many participants lost more than one-fifth of their own weight. This data not only significantly surpasses predecessors such as semaglutide and Tirzpetide, but more importantly, it brings the dream of "drugs approaching the effect of weight loss surgery" into reality. For billions of obese individuals, especially those with severe complications who are unable or unwilling to undergo surgery, the formulation made from Retatrutide powder represents a new beacon of hope. Its weight loss mechanism is a multi pronged approach: through the synergistic effect of GLP-1R and GIPR, it strongly inhibits appetite, delays gastric emptying, and increases satiety; At the same time, by activating GCGR, energy consumption is increased, promoting the conversion of white fat to beige fat, that is, "burning" fat. This combination of "opening up sources and reducing costs" is the cornerstone of achieving excellent weight loss effects.
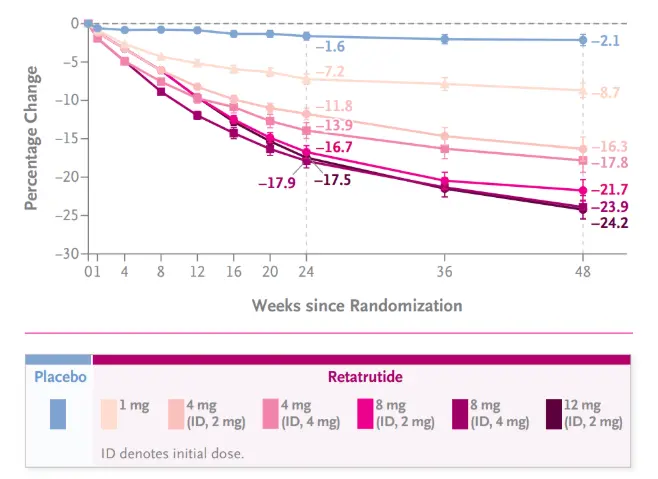
Secondly, in the management of type 2 diabetes (T2DM), it is expected to achieve "steady cruise" of blood sugar. Traditional hypoglycemic drugs often focus on a single mechanism, while Retarutide provides comprehensive blood glucose management. ① Through GLP-1R and GIPR, precise regulation is achieved when blood sugar levels rise, while the effect weakens when blood sugar levels are normal, greatly reducing the risk of hypoglycemia. This is an "intelligent" hypoglycemic mode. ② Although it is an agonist of GCGR itself, it can inhibit inappropriate postprandial glucagon elevation and reduce hepatic glucose output in the overall physiological environment. ③ Significant weight loss effects and the GIPR signaling pathway itself can greatly improve the sensitivity of peripheral tissues such as muscles and fat. Clinical data shows that Retarutide can lead to a decrease in glycated hemoglobin (HbA1c) of up to 2.0% or more, accompanied by multiple benefits such as weight, blood lipids, and blood pressure. This marks a new era in the treatment of T2DM with "cardiovascular metabolic comprehensive benefits" as its core.
Furthermore, its potential therapeutic effect on non-alcoholic steatohepatitis (NASH) is exciting. NASH is a progressive form of fatty liver characterized by hepatic steatosis, inflammation, and fibrosis, and there are currently no approved effective drugs. The triple mechanism of action of Retatrutide precisely targets multiple core components of NASH: potent weight loss and GCGR activation can directly reduce liver fat deposition; Its anti-inflammatory effect helps alleviate liver inflammation; Preclinical studies also suggest that it may directly inhibit the activation of hepatic stellate cells, thereby delaying or even reversing the process of liver fibrosis. Although this indication is still in clinical research, it has shown great potential to become a core therapy for NASH.
In addition, its comprehensive management of cardiovascular risks (improving blood lipids, blood pressure) and improvement of polycystic ovary syndrome (PCOS) constitute a part of its broad application prospects. Therefore, the value of Retatrutide raw powder lies in its ability as a core ingredient to be made into a "weapon" to tackle a range of the most common and challenging chronic metabolic diseases, and its market ceiling is far from being reached.
Mechanism of Action: A Subtle Metabolic Trio
To understand why Retatrutide powder is so powerful, we must delve into the microscopic world of molecules and cells, and appreciate how it commands a sophisticated metabolic 'trio'. This is not a simple superposition of three signals, but a highly coordinated and sometimes antagonistic precise ensemble, with the ultimate effect being a metabolic regulation miracle of 1+1+1>3.
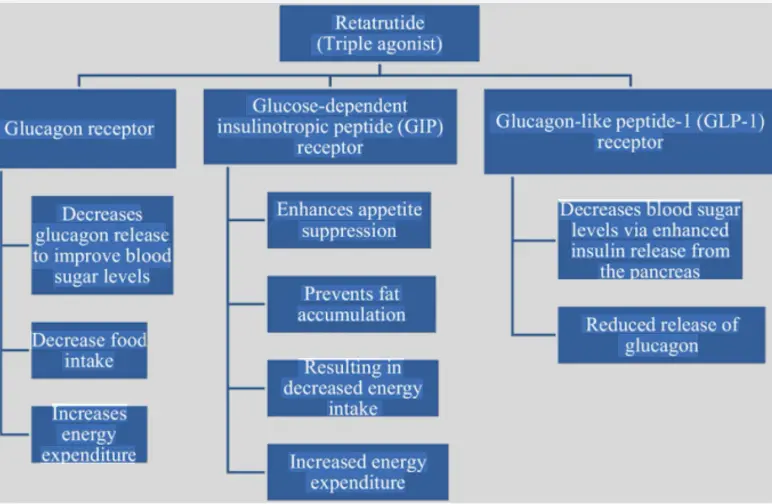
First movement: GLP-1 receptor activation - the "conductor" who sets the tone
GLP-1 is the "intestinal glucagon" secreted by intestinal L cells, which is the core signal for regulating blood sugar and energy in the body. Retatrutide plays a robust conductor role by activating GLP-1 receptors. In the brain, it mainly acts on the appetite center of the hypothalamus, emitting strong signals of "satiety" while inhibiting the activity of "hunger" neurons, thereby reducing energy intake from the source. In the pancreas, it listens to the melody of "blood glucose concentration" and accurately commands beta cells after meals, while directing alpha cells to reduce the secretion of glucagon, achieving a smooth drop in blood sugar. In the gastrointestinal tract, it acts as a "decelerator", delaying gastric emptying, allowing food to be slowly absorbed, further smoothing postprandial blood sugar fluctuations, and prolonging satiety. This is the cornerstone of Retarutide's mechanism of action and an important guarantee of its safety.
Second movement: GIP receptor activation - the "reconciler" who breaks the deadlock
GIP is another important incretin, but its role in T2DM patients has long been controversial and was once considered "ineffective". The innovation of Retatrutide lies in its reactivation of the GIP pathway. The action of GIP receptor agonists is like a clever 'moderator'. Firstly, it can synergize with GLP-1 to further enhance glucose dependent insulin secretion, especially in hyperglycemic conditions. Secondly, and more importantly, GIP has a unique effect on adipose tissue. Research has found that activation of GIPR can enhance the inhibitory effect of GLP-1R in the feeding center and may promote energy storage in adipose tissue. However, the overall effect of Retarutide is strong weight loss, suggesting that the metabolic effects of GIP are reprogrammed in the presence of GCGR. It may improve the distribution and utilization of energy rather than simply promoting storage. In addition, GIP signaling is believed to alleviate gastrointestinal adverse reactions (such as nausea and vomiting) caused by GLP-1, improve drug tolerance, and enable Retatrutide to be accepted by more patients while achieving high efficacy.
Third movement: Activation of glucagon receptors - the "engine" that ignites energy.
This is the most unique and daring design of Ritaglutide. Glucagon has traditionally been regarded as a glucose raising hormone, so why can stimulating its receptors actually lead to weight loss and blood sugar reduction? This is precisely the scientific subtlety of it. The activation of GCGR plays the role of an "energy engine" here. ① Significantly increase energy expenditure: It acts on the liver to increase the level of the active form T3 of Calcitonin, thereby increasing the basal metabolic rate of the whole body, allowing you to burn more calories even in a resting state. ② Promoting fat burning: The activation of GCGR can strongly promote the β - oxidation of fatty acids in the liver and redirect energy from white adipose tissue (storing energy) to beige adipose tissue (consuming energy), a process known as "fat thermogenesis". ③ Regulating amino acid metabolism: Glucagon signaling is closely related to protein metabolism, which may help maximize muscle mass retention during weight loss and achieve the ideal effect of "reducing fat and preserving muscle".
The success of this' trio 'lies in its perfect internal balance.
GLP-1 and GIP are responsible for "throttling" (reducing intake), while GCGR is responsible for "opening up" (increasing consumption). GIP alleviates gastrointestinal discomfort caused by GLP-1, while the thermogenic effect of GCGR is constrained by the hypoglycemic and satiety effects of GLP-1/GIP, avoiding excessive glucose elevation. The three mutually constrain and promote each other, jointly pushing metabolic balance towards a new and healthier state. Therefore, the mechanism of action of Retatrutide is a deep and synergistic reprogramming of the body's energy homeostasis.

Comparison: How can one claim the title of king in the "League of Kings"?
In the "King Alliance" of GLP-1 drugs, Retarutide is a latecomer but a formidable challenger. Comparing it with the current market leader can more clearly position the unique value of its original fans.
VS Semaglutide, GLP-1 single target
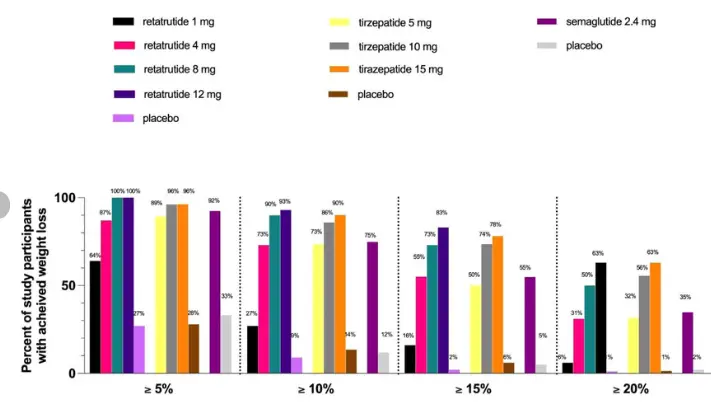
Semaglutide is a hero who opened up the era, pushing the efficacy of GLP-1 single target to the extreme. However, the comparison between Retatrutide and it is like the difference between a "multifunctional Swiss Army knife" and an "elite tactical dagger". In terms of key weight loss indicators, Semaglutide achieved a historic breakthrough of approximately 15% weight loss at week 68; But Retatrutide achieved a decrease of over 24% within 48 weeks, taking the efficacy to a whole new level. In terms of blood sugar reduction, both are excellent, but Retarutide relies on the synergistic effect of GIP and the increased energy consumption brought by GCGR. From the perspective of its mechanism of action, Semaglutide is a precise single point breakthrough, while Retatrutide is a comprehensive positional warfare.
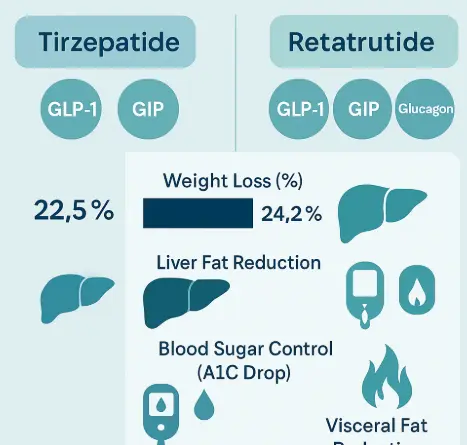
VS Tirzepatide, GLP-1/GIP dual targets
Tirzepatide is the most direct "predecessor" of Retarutide, which proves the superiority of the dual target strategy, and its weight loss effect (about 21%) has surpassed semaglutide. The comparison between Retatrutide and Tirzepatide is a contest between "duet" and "trio". The advantage of Tirzepatide lies in its first mover advantage and market validation. But the third target increased by Retatrutide, GCGR, constitutes a key differentiation. This' energy engine 'is likely the core reason for Retatrutide's further breakthrough in weight loss effect. It can be analogized as Tirzepatide creating a huge calorie deficit by strongly suppressing appetite and intelligently lowering blood sugar; On this basis, Ritaglutide also "ignited the fire of the body" and actively burned more fat. Of course, the addition of GCGR may also bring some potential challenges, such as mild increase in heart rate (within clinically controllable range), but the therapeutic benefits it brings currently seem overwhelming.
VS Future Competitors (such as CagriSema, etc.)
CagriSema is a compound formulation of semaglutide and the long-acting amygdalin analogue Cagralintide, which also demonstrates great potential. It works synergistically through GLP-1 and amylin (another potent appetite suppressant signal). Compared to Retarutide, this represents another technological pathway: a "cocktail therapy" that combines different molecules versus a single molecule of "multiple agonists". Single molecules may have advantages in production processes, quality control, and immunogenicity risks; Compound preparations may be more flexible in adjusting dosage ratios. At present, there is a lack of direct head to head comparison data, but Retarutide undoubtedly holds a leading position in scientific innovation and therapeutic potential due to its inherent and precisely designed synergistic mechanism.
Research prospects: From metabolic diseases to broader therapeutic territories
The story of Retatrutide has just begun its first chapter. Based on its unique and powerful mechanism of action, its research prospect is far beyond obesity and type 2 diabetes, and is expanding to a broader therapeutic domain.
1. Breakthrough therapy for NASH/metabolism related fatty liver disease (MASLD): This is the next most likely major indication for Retatrutide to be approved. Its potential for "weight loss+direct anti-inflammatory and anti fibrotic effects" makes it the first drug capable of reversing liver fibrosis. The current large-scale phase III clinical trials are verifying this hypothesis. If successful, it will leverage a huge, unmet healthcare market.
2. Cardiovascular Outcome Study (CVOT) and Heart Failure: Like all heavyweight metabolic drugs, the cardiovascular outcome trial of Retarutide is crucial. We not only expect it to demonstrate cardiovascular safety like semaglutide, but also look forward to its special value in the treatment of heart failure, especially heart failure HFpEF with preserved ejection fraction, which is closely related to obesity. Its significant weight loss and diuretic effects (possibly related to GCGR activation) may bring pathological and physiological benefits to HFpEF patients.
3. Exploration of central nervous system (CNS) diseases: Increasing evidence suggests that metabolic signals are closely related to neurodegenerative diseases. GLP-1 receptor agonists have shown signs of delaying disease progression in clinical studies of Alzheimer's disease and Parkinson's disease. Can Retatrutide go further in the field of neuroprotection with its stronger signal activation ability and multi-target properties? This is a highly attractive exploration direction.
4. Comprehensive management of kidney disease and metabolic syndrome: obesity and diabetes are the main driving factors of chronic kidney disease (CKD). Can Retatrutide replicate or even surpass the success of its predecessors in delaying the progression of CKD? Meanwhile, for metabolic syndrome that combines obesity, hypertension, dyslipidemia, and hyperglycemia, Retatrutide is an ideal candidate for "targeted treatment" and is expected to achieve "multiple treatments with one drug".
5. Innovation in dosage forms and administration methods: From the perspective of active pharmaceutical ingredients, future research also includes optimization of dosage forms. Developing oral dosage forms (although highly challenging for peptides), or longer lasting monthly or even longer lasting formulations, will be the key to improving patient convenience and market competitiveness. In addition, exploring the efficacy and safety in different populations, such as adolescent obesity and hereditary obesity syndrome, will also enrich their application scenarios.
Conclusion
Peptide powder Retatrutide, as an outstanding representative of the "triple agonist", it is not an accidental discovery, but the crystallization of rational design and technological breakthroughs based on a profound understanding of metabolic physiology. It stands at the forefront of the metabolic therapy revolution with its exquisite molecular structure, unprecedented therapeutic effects, synergistic and complementary mechanisms of action, and unlimited imagination for future treatments. For the field of pharmaceutical raw materials, it represents a new benchmark; For clinical doctors, it is an upcoming 'super weapon'; For billions of patients, it is a stronger dawn that illuminates a healthy future. The treatment pattern of metabolic diseases is being profoundly and permanently reshaped due to its emergence.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality Peptide powder Retatrutide, that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of Peptide Retatrutide, please contact our technical team through the following methods:sales12@faithfulbio.com.
Reference
1. Rosenstock, J., et al. (2023). Retatrutide, a GIP, GLP-1 and Glucagon Receptor Agonist, for People with Type 2 Diabetes: A Randomised, Double-Blind, Placebo and Active-Controlled, Phase 2 Trial. The Lancet, 402(10466), 529-544.
2. Jastreboff, A. M., et al. (2023). Retatrutide for the Treatment of Obesity: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study. Nature Medicine, 29, 2923–2932.
3. Finan, B., et al. (2022). The GIP/GLP-1/Glucagon Triple Agonist Retatrutide Mediates Robust Body Weight Loss and Improved Glycemic Control in Preclinical Models. Molecular Metabolism, 64, 101558.
4. Müller, T. D., et al. (2019). The New Biology and Pharmacology of Glucagon. Physiological Reviews, 99(2), 945-1385.
5. Coskun, T., et al. (2018). The Pharmacology and Therapeutic Potential of the GIP, GLP-1 and Glucagon Receptor Co-agonism. Molecular Metabolism, 18, 1-11.
6. Frias, J. P., et al. (2018). Efficacy and Safety of LY3298176, a Novel Dual GIP and GLP-1 Receptor Agonist, in Patients with Type 2 Diabetes: A Randomised, Placebo-Controlled and Active Comparator-Controlled Phase 2 Trial. The Lancet, 392(10160), 2180-2193.
7. Samms, R. J., et al. (2021). How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends in Endocrinology & Metabolism, 32(6), 345-357.



