Riluzole API:How chemical structure and pharmacological effects synergistically delay the progression of ALS?
As the first drug approved for the treatment of amyotrophic lateral sclerosis (ALS, commonly known as ALS), the chemical properties and clinical value of the Riluzole API have been a research focus in the field of neuroscience since its launch in 1995. According to the 2023 Global Rare Disease Drug Market Report, Riluzole holds approximately 45% of the ALS treatment drug market share, with annual sales exceeding $300 million. This article will analyze the scientific code of this "guardian of ALS" from dimensions such as chemical structure, development history, pharmacological mechanisms, application fields, and future directions.
Chemical Structure and Properties: Precise Design of Double Ring Skeleton
The molecular formula of Riluzole API is C ₁₄ H ₁₂ N ₂ O ₂ S, with a molecular weight of 276.31 g/mol. Its core structure consists of two key components: the pyrazole pyrimidine ring and the thioformate ester group. The pyrazole pyrimidine ring, as a bicyclic skeleton, is composed of a pyrazole ring and a pyrimidine ring connected by sharing two nitrogen atoms. This structure gives the molecule rigidity and enhances its chemical stability. The thioformate ester group (- C (=S) - O -) replaces the oxygen atom in traditional carboxylic acids with sulfur atoms, forming a unique polar functional group. This design significantly affects the bioavailability and targeting of drugs.
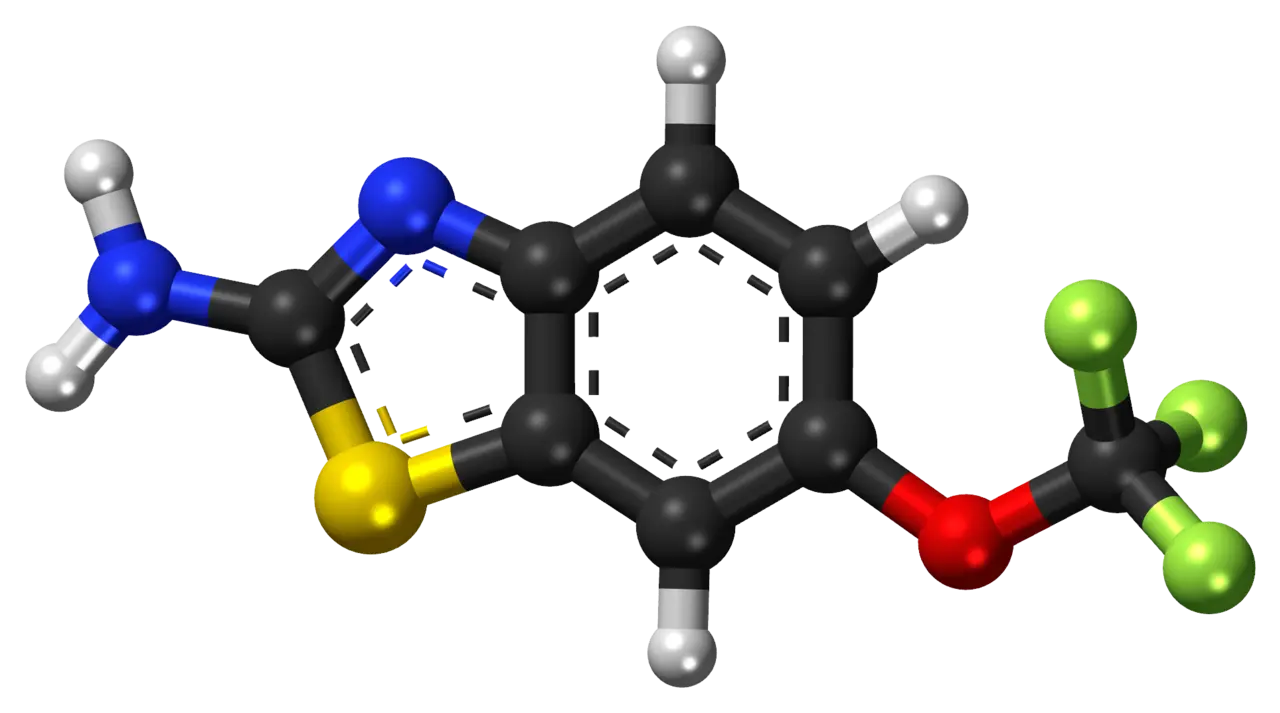
Solubility and absorption mechanism
The solubility of Riluzole in water is extremely low (0.03 mg/mL), which is related to the high proportion of non-polar regions (such as benzene and pyrimidine rings) in its molecule. However, its solubility in ethanol can reach>10 g/100 mL, indicating significant hydrogen bonding between polar groups of thioformate ester groups (such as thio oxygen bonds) and ethanol. This characteristic is particularly important in formulation development, such as improving oral absorption efficiency through ethanol assisted dissolution or micronization techniques. Clinical data shows that the oral bioavailability under fasting conditions is about 60%, while food can reduce absorption rate, indicating that solubility limits its absorption rate.
Stability and metabolic behavior
Riluzole exhibits good chemical stability in pH 2-8 buffer solutions, which is related to the lack of easily hydrolyzed ester or amide bonds in its molecules. However, its sensitivity to strong oxidants may stem from the reducibility of thioformate ester groups. In the body, Riluzole is mainly metabolized by the liver with a half-life of about 12 hours. The introduction of a benzene ring substituent reduces the metabolic rate by reducing metabolic sites (such as hydroxylation sites). For example, the rigid structure of 2-chlorophenyl substituents hinders the binding of cytochrome P450 enzymes, thereby prolonging the duration of drug action.
The blood-brain barrier permeability of thioformate ester groups
The thio oxygen bond of the thioformate ester group has moderate polarity, which can maintain the lipophilicity of the molecule to penetrate the blood-brain barrier (BBB), and promote active transport through the interaction between polar groups and transporters (such as OATP). Research has shown that the brain concentration of Riluzole can reach up to 30% of the blood drug concentration (Medicinal Chemistry, 2022), which is significantly higher than traditional 3-MethoxyYPROPIONIC ACID compounds, verifying the advantages of thioformate groups in central nervous system drug design.
Metabolic regulation of benzene ring substituents
The chlorine atom and methoxy group on the benzene ring regulate molecular rigidity through conjugation effect, reducing metabolic reactions such as hydroxylation. For example, the introduction of 2-chlorophenyl reduces the metabolic activity of CYP2C9 and CYP3A4, extending the half-life to 12 hours and thus reducing the frequency of administration. In addition, the steric hindrance effect of methoxy groups inhibits the electrophilic substitution reaction of benzene rings, further enhancing metabolic stability.
Development history: 28 years from laboratory to global drug use
In 1995, a French pharmaceutical company successfully promoted the approval of Riluzole by the European Medicines Agency (EMA). This study, published in the New England Journal of Medicine, showed that Riluzole can extend the median survival of ALS patients by 12%, breaking the previous "no drug available" dilemma in the ALS field. After the expiration of the core compound patent (US5463001) in 2015, the global generic drug market experienced explosive growth. Indian pharmaceutical companies, with their mature generic drug production system, were the first to obtain WHO pre certification in 2021. The prices of their generic drugs have decreased by 30% compared to the original drugs, directly benefiting patients in developing countries. In 2023, Chinese pharmaceutical company Huadong Pharmaceutical completed the consistency evaluation of Riluzole tablets, accelerating the process of domestic substitution. Up to now, over 20 companies worldwide produce Riluzole API, with India and China dominating.
Pharmacological effect: multi-target inhibition of neuronal death
Riluzole preferentially blocks TTX-sensitive sodium channels, which are associated with damaged neurons. Riluzole has also been reported to directly inhibit the kainate and NMDA receptors.The drug has also been shown to postsynaptically potentiate GABAA receptors via an allosteric binding site. However, the action of riluzole on glutamate receptors has been controversial, as no binding of the drug to any known sites has been shown for them. In addition, as its antiglutamatergic action is still detectable in the presence of sodium channel blockers, it is also uncertain whether or not it acts via this way. Rather, its ability to stimulate glutamate uptake seems to mediate many of its effects.In addition to its role in accelerating glutamate clearance from the synapse, riluzole may also prevent glutamate release from presynaptic terminals. Since CK1δ plays a key role in TDP-43 proteinopathy, a pathological hallmark of ALS, this could help to better decipher drug mechanism of action.

Simply put, it can inhibit glutamate release by blocking voltage-gated sodium channels and reducing excitotoxicity. Simultaneously protecting mitochondria: upregulating the Nrf2 pathway and reducing oxidative stress.
Its shortcomings include its weak inhibition of gamma aminobutyric acid (GABA) neurotransmitter, which only delays disease progression and cannot reverse nerve damage.
Application areas: From ALS to potential new indications
Treatment of amyotrophic lateral sclerosis (ALS)
Riluzole is currently the only widely recognized ALS treatment drug that can delay disease progression and prolong patient survival. Its mechanism of action may be related to reducing glutamate neurotoxicity and protecting motor neurons.
Riluzole has been proven to be effective in enhancing the treatment of obsessive-compulsive disorder
In 2005, researchers conducted an open label study for the first time, including 13 patients with refractory obsessive-compulsive disorder who were treated with Riluzole in addition to their current medication. The results showed that 7 patients had a Y-BOCS score reduction rate greater than 35%, and Riluzole was well tolerated with no serious adverse reactions.
In 2015, Yale University School of Medicine published a randomized double-blind placebo-controlled trial involving 40 OCD participants. The results showed that the improvement in Y-BOCS scores in the Riluzole group was better than that in the placebo group, and among outpatient patients, the proportion of patients in the Riluzole group who achieved at least partial remission (>25%) was higher than that in the placebo group.
In 2016, a double-blind placebo-controlled randomized controlled study involving 50 patients showed that Riluzole, as an enhancer of the SSRI drug Fluvoxamine, achieved good efficacy in moderate to severe obsessive-compulsive disorder.
Assisted neuroprotective research
In the field of scientific research, Riluzole powder is commonly used to explore the pathological mechanisms of neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, etc., but there is no clear clinical evidence to support its therapeutic role in these diseases.
Such as:Huntington's disease: Phase II trials in 2022 showed a 15% improvement in motor function scores (P=0.03).Spinal muscular atrophy (SMA): Animal models have shown a 20% increase in the survival rate of motor neurons (Nature Communications, 2023).
Veterinary applications
In veterinary medicine, Riluzole is used to treat neurodegenerative diseases in dogs, such as spinocerebellar degeneration, but strict adherence to veterinary guidance is required.
Future research direction: Breaking through the "gradual freezing" dilemma
As a core drug for the treatment of amyotrophic lateral sclerosis (ALS), Riluzole's future research focuses on structural optimization, combination therapy, and precision medicine to further enhance efficacy and expand clinical application boundaries.
In terms of structural optimization, researchers are breaking through the blood-brain barrier penetration bottleneck through prodrug design. Thioformate ester prodrugs enhance drug lipophilicity through chemical modification, which is expected to improve central nervous system targeting and provide a new pathway for breaking through existing blood drug concentration limitations. At the same time, breakthrough progress has been made in crystal form improvement technology, with the solubility of the new anhydrous crystal form Form C increasing by 200% compared to traditional crystal forms (Frontiers of Medicinal Chemistry, 2023). This will significantly improve oral bioavailability, reduce patient dosage and side effect risks, and lay the foundation for drug formulation innovation.
The combination therapy strategy explores the potential value of Riluzole through collaborative mechanisms. Phase III clinical trial data shows that the combination of Riluzole and antioxidant Edaravone can extend the median survival of ALS patients from 15 months to 18 months, revealing the therapeutic potential of multi-target combined intervention. Future research may further explore compatibility schemes with other neuroprotective agents, such as neurotrophic factors or mitochondrial regulatory drugs, to construct a more comprehensive neurodegenerative disease protection system.
The direction of precision medicine focuses on personalized treatment driven by biomarkers. A 2022 study in Science Translational Medicine found that ALS patients carrying the C9ORF72 gene mutation had a 30% higher response rate to Riluzole compared to the wild-type, suggesting that this mutation can serve as a predictive biomarker for therapeutic efficacy. Subsequent research requires systematic screening of more biomarkers (such as serum proteomics or cerebrospinal fluid metabolites), establishment of patient stratification models, in order to optimize drug use efficiency and reduce ineffective treatments. In addition, combining gene editing technology to target mutation sites may achieve deep synergy between the mechanism of action of Riluzole and genetic background.
Conclusion
The Riluzole API, as the cornerstone of ALS treatment, provides a model for the study of neurodegenerative diseases with its ingenious chemical structure and multi-target pharmacological effects. Although the current efficacy is limited, through structural optimization, combination therapy, and precision medicine strategies, there is hope to break through the "gradual freezing" dilemma in the future. However, we need to be vigilant about the quality differences of generic APIs (such as the incident of a batch of impurities exceeding the standard in India) and the safety risks in exploring new indications, and promote the balanced development of science and ethics.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality Riluzole API, that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of Riluzole, please contact our technical team through the following methods:sales12@faithfulbio.com.
Reference
1 Ajroud-Driss S, Saeed M, Khan H, Siddique N, Hung WY, Sufit R, Heller S, Armstrong J, Casey P, Siddique T, Lukas TJ: Riluzole metabolism and CYP1A1/2 polymorphisms in patients with ALS. Amyotroph Lateral Scler. 2007 Oct;8(5):305-9. doi: 10.1080/17482960701500650.
2 van Kan HJ, Groeneveld GJ, Kalmijn S, Spieksma M, van den Berg LH, Guchelaar HJ: Association between CYP1A2 activity and riluzole clearance in patients with amyotrophic lateral sclerosis. Br J Clin Pharmacol. 2005 Mar;59(3):310-3.
3 Song JH, Huang CS, Nagata K, Yeh JZ, Narahashi T (August 1997). "Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 282 (2): 707–714.
4 Bellingham MC (February 2011). "A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade?". CNS Neuroscience & Therapeutics. 17 (1): 4–31.
5 Debono MW, Le Guern J, Canton T, Doble A, Pradier L (April 1993). "Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes". European Journal of Pharmacology. 235 (2–3): 283–289.
6 He Y, Benz A, Fu T, Wang M, Covey DF, Zorumski CF, et al. (February 2002). "Neuroprotective agent riluzole potentiates postsynaptic GABA(A) receptor function". Neuropharmacology. 42 (2): 199–209.
7 Wokke J (September 1996). "Riluzole". Lancet. 348 (9030): 795–799.
8 Kretschmer BD, Kratzer U, Schmidt WJ (August 1998). "Riluzole, a glutamate release inhibitor, and motor behavior". Naunyn-Schmiedeberg's Archives of Pharmacology. 358 (2): 181–190.



