Rosuvastatin Calcium API powder:How to shake the global defense against cardiovascular disease?
In the protracted war between human beings and atherosclerotic cardiovascular diseases, statins are undoubtedly the most brilliant "generals". Among them, Rosuvastatin Calcium is known as the "super statin" for its excellent efficacy and safety, and has quickly become a cornerstone of lipid-lowering treatment since its introduction in 2003. However, when we focus on pills or capsules, we often overlook the true source of their power - active pharmaceutical ingredients. Rosuvastatin Calcium API powder,as the active core and efficacy cornerstone of drugs, the wisdom of their molecular design, exquisite production processes, and strict quality control have jointly written the legend of modern pharmaceutical science. This article will delve into the microscopic world of active pharmaceutical ingredients, from molecular structure to clinical applications, and comprehensively analyze how Rosuvastatin Calcium API powder has transformed from a laboratory molecular concept to a "chemical guardian" safeguarding the heart health of millions of patients worldwide.
Molecular Structure: Carefully crafted 'molecular key' unlocks the door to efficient lipid-lowering
The molecular structure of Rosuvastatin Calcium is not a gift from nature, but a masterpiece of rational design by medicinal chemists based on a deep understanding of the target. Its full name is (3R, 5S, 6E) -7- [4- (4-Fluorophenyl) -6-isopropyl-2- [methyl (methylsulfonyl) amino] pyridinin-5-yl]) -3,5-dihydroxyhept-6-enoic acid chemical salt. This seemingly lengthy name actually accurately describes every tooth mark of it as a "high-precision key".
Core Structure Analysis and Design Philosophy:
Hydrophilic pyrimidine ring and methanesulfonamide group - precise anchor point for targeted binding: Unlike early lipophilic statins such as Lovastatin and Simvastatin, Rosuvastatin's core is a pyrimidine ring with a methanesulfonamide group. This design is crucial. The methanesulfonamide group can form a strong hydrogen bonding network and polar interactions with specific amino acid residues in the active pocket of HMG CoA reductase, such as Lys735 and Ser684, with a binding affinity approximately 10000 times stronger than the natural substrate HMG CoA. This is like upgrading an iron key to a specially designed key that perfectly matches the internal texture of the lock cylinder, achieving almost irreversible strong competitive suppression. Meanwhile, the hydrophilic pyrimidine ring structure reduces the ability of drugs to passively diffuse through the cell membrane, which is the structural basis for its high liver selectivity.
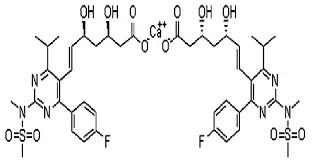
(3R,5S)-3, 5-dihydroxyheptanoic acid side chain - mimicking the natural "fraud master": This side chain is the key to Rosuvastatin's ability to "impersonate" the real substrate of HMG CoA reductase - HMG CoA. Its three-dimensional configuration (3R, 5S) is highly similar to the reduced state of HMG CoA, allowing it to perfectly embed into the active site of the enzyme, but it cannot be further catalyzed by the enzyme, thus "blocking" the cholesterol synthesis production line. This clever 'molecular imitation' is the direct reason for its high efficiency.
Calcium salt form - a "wise choice" for stability and delivery: Rosuvastatin exists in calcium salt form, which is not accidental. Firstly, salt formation significantly improves the crystallinity and physical stability of the compound, making the raw powder less prone to moisture absorption or degradation during storage and transportation. Secondly, the calcium salt form has better solubility in neutral to weakly alkaline environments in the gastrointestinal tract, which is beneficial for drug release and absorption. According to research, the absolute bioavailability of its calcium salt form is about 20%. Although not high, combined with its extremely strong potency, it fully meets the therapeutic needs.
In the history of drug development, from Fluvastatin (the first fully synthetic statin) to Atorvastatin, and then to Rosuvastatin, the introduction of hydrophilic groups and continuous optimization of target binding affinity are clear main lines. A study published in the Journal of Medicinal Chemistry visually demonstrated through X-ray crystallography that Rosuvastatin forms up to 8 hydrogen bond interactions with the active site of HMG CoA reductase, while early statins such as simvastatin can only form 3-4, which directly explains why they have higher inhibitory efficacy (lower IC50 value) from a structural perspective.
Characteristics: The quality of the raw powder is the "star setting" for the excellent performance of the formulation
As an active pharmaceutical ingredient, the physicochemical and quality properties of Rosuvastatin Calcium API powder are fundamental to determining the safety, efficacy, and consistency of the final drug. Its quality control is far from being summarized by the four words' white powder ', but rather a collection of precise scientific parameters.
Polycrystalline phenomenon and solid-state properties: Rosuvastatin Calcium is known to exist in multiple crystal forms (such as crystal forms I, II, etc.). Different crystal forms may have differences in solubility, density, hardness, and dissolution rate. For example, a certain metastable crystal form may dissolve faster initially, but may transition to a stable crystal form during long-term storage, resulting in changes in product performance. Therefore, the production of active pharmaceutical ingredients must strictly lock in and stabilize the thermodynamically stable crystal form (usually crystal form I) required for production, ensuring consistency in the dissolution behavior from raw powder to finished tablets. This needs to be achieved by controlling process parameters such as crystallization solvent, cooling rate, and stirring intensity.
Chiral purity - a mirror error that life cannot tolerate: The 3,5-dihydroxy side chain of rosuvastatin contains two chiral centers, and only the (3R, 5S) configuration has strong pharmacological activity. Its enantiomers or diastereomers are not only ineffective, but may also pose unpredictable safety risks. The synthesis process must use highly stereoselective asymmetric synthesis techniques (such as using chiral catalysts or chiral pool materials) to ensure extremely high optical purity of the final raw powder, typically requiring an excess of enantiomers (e.e. value) greater than 99.5%. Chiral high-performance liquid chromatography (HPLC) is required for strict monitoring during the production process.
Mass spectrometry control - a "hidden battlefield" related to safety: impurities in raw materials mainly come from starting materials, intermediates, side reaction products, and degradation products in the synthesis route. Especially the presence of N-Methylmethanesulfinamide groups requires attention to the risk of potential genotoxic impurities in the process. These impurities may pose safety hazards even at the ppm (parts per million) level.
Working principle: Implementing precise blasting in the "production workshop" of liver cells
The role of Rosuvastatin Calcium is not simply "inhibiting enzyme activity", but rather a biological process that occurs in liver cells and is finely regulated at multiple levels.
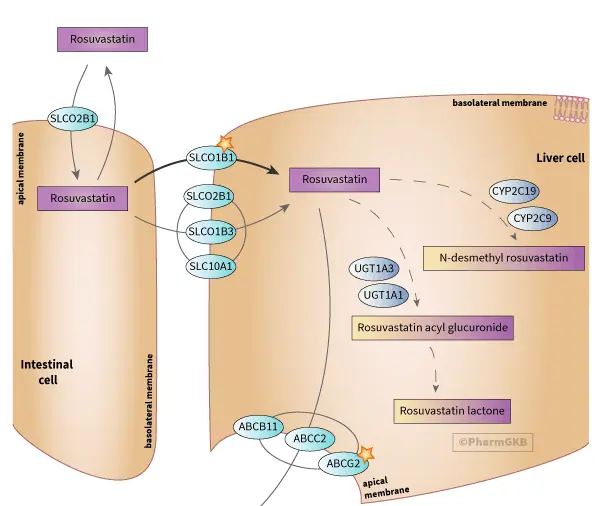
Active transport and liver first pass effect: After use, Rosuvastatin Calcium is absorbed in the intestine. Due to its hydrophilicity, it cannot freely diffuse into various tissues and cells throughout the body like liposoluble statins. It mainly relies on the active uptake of organic anion transport peptide 1B1 (OATP1B1) highly expressed on the basement membrane of liver cells. The high selectivity of this transporter allows about 70-80% of the drug to enter the liver, with only a small amount entering the peripheral circulation. This brings two major advantages: firstly, it greatly improves liver targeting, increases local drug concentration, and enhances therapeutic efficacy; Secondly, it significantly reduces the exposure of drugs to non target tissues such as muscles, which is an important reason for the relatively low incidence of muscle toxicity side effects (such as muscle pain and rare rhabdomyolysis). After entering liver cells, some drugs are initially metabolized by metabolic enzymes (such as CYP2C9, which has slight metabolism), further reducing the amount of drugs entering the systemic circulation.
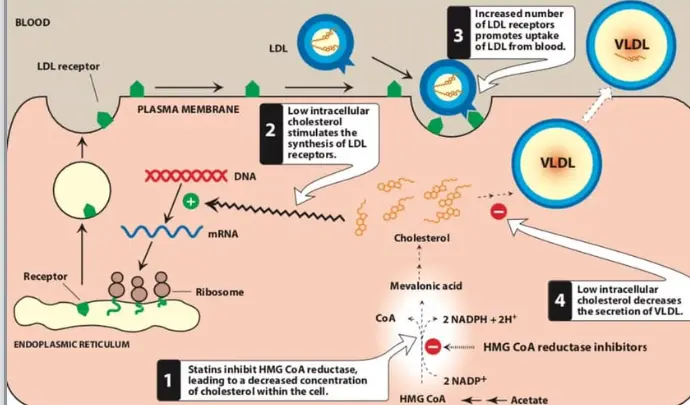
Locking inhibition of HMG CoA reductase: After entering liver cells, the active acid form of Rosuvastatin Calcium (calcium salt dissociates in vivo) binds to HMG CoA reductase. Its binding affinity (Ki value of approximately 5.4 pM) far exceeds that of natural substrates. This strong inhibition blocks the 3,5-dihydroxy-3-methyl-Pentanoic acid pathway in the early stages. 3,5-dihydroxy-3-methyl-Pentanoic acid is not only a precursor of cholesterol, but also a precursor of a series of important Isoprene derivatives such as Coenzyme Q10 and farnesyl pyrophosphate. However, the therapeutic dose of Rosuvastatin Calcium mainly affects cholesterol synthesis, with relatively little interference on other pathways.
Triggering the compensatory scavenger response of liver cells: a sharp decrease in cholesterol synthesis within liver cells is perceived by the cells as a "cholesterol deficiency". As feedback regulation, the steroid regulatory element binding protein (SREBP) pathway in the nucleus is activated. SREBP is transported to the Golgi apparatus and cleaved before entering the nucleus, upregulating the expression of low-density lipoprotein receptor (LDLR) genes. So, a large number of newly synthesized LDLRs were deployed on the surface of liver cells. These receptors act like a vacuum cleaner, forcefully capturing low-density lipoprotein particles in the blood and engulfing them into liver cells for breakdown and metabolism. This is the main mechanism by which Rosuvastatin Calcium reduces blood LDL-C levels, contributing over 80%. Research has shown that Rosuvastatin Calcium can increase the number of LDLR by several times.
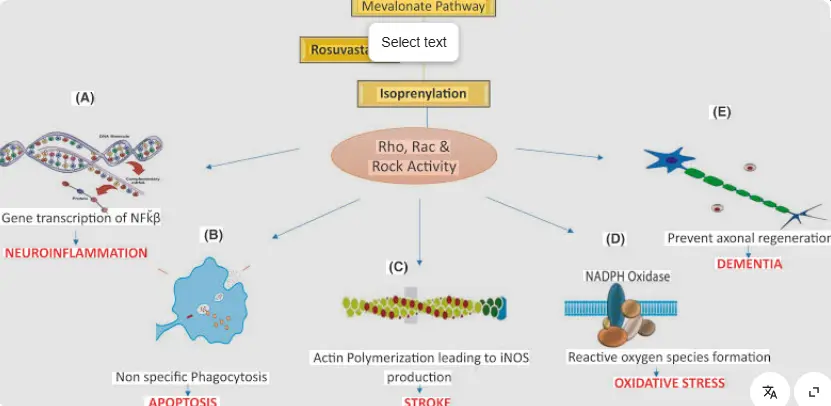
Beyond lipid-lowering: possible contribution of pleiotropic effect: in addition to powerful lipid-lowering, Rosuvastatin Calcium has also been observed to have "pleiotropic" effects such as improving endothelial function, anti-inflammatory, and stabilizing atherosclerotic plaque. These effects are partly due to its inhibition of downstream products of the 3,5-dihydroxy-3-methyl-Pentanoic acid pathway, thereby affecting intracellular signal transduction. For example, reducing the farnesylation of small G proteins may inhibit inflammatory pathways. The famous JUPITER study (2008) found that Rosuvastatin Calcium can significantly reduce the risk of major cardiovascular events in patients with normal LDL-C levels but elevated high-sensitivity C-reactive protein, suggesting that its anti-inflammatory effect may be independent of lipid-lowering and exert additional protective effects.
Purpose: From blood lipid management to comprehensive defense against cardiovascular events
The ultimate value of Rosuvastatin Calcium API powder is realized through its widespread clinical application. Its use has expanded from simple lipid regulation to primary and secondary prevention of cardiovascular diseases.
Specific clinical applications and evidence-based medicine evidence:
Primary hypercholesterolemia and mixed dyslipidemia: these are its most classic indications. For patients with ineffective dietary control, Rosuvastatin Calcium can dose dependently reduce total cholesterol (TC), LDL-C, and apolipoprotein B (ApoB), while moderately increasing high-density lipoprotein cholesterol (HDL-C). Its LDL-C reduction rate ranks among the top among similar drugs, with a dose of 10 mg reducing LDL-C by approximately 45% -50%.
Secondary prevention of atherosclerotic cardiovascular disease: For patients who have suffered from coronary heart disease, myocardial infarction, stroke or peripheral artery disease, no matter what their baseline blood lipid level is, the use of Rosuvastatin Calcium to strengthen lipid reduction to stabilize/reverse plaque and prevent recurrence of events has become the gold standard. ASTEROID test (2006) confirmed by intravascular ultrasound that the volume of atherosclerotic plaque can be significantly reversed (median reduction 0.79%) after 2 years of treatment with Rosuvastatin Calcium, which is the first large-scale study directly proving that statins can reverse plaque.
Primary prevention and risk assessment: For the population without clinical cardiovascular disease but with multiple risk factors (such as hypertension, diabetes, smoking, family history of early onset cardiovascular disease), Rosuvastatin Calcium is used for primary prevention. The JUPITER study, as mentioned earlier, provides strong evidence for primary prevention in a specific population (with elevated inflammatory markers but low LDL-C), expanding the scope of statin use.
Other exploratory applications: Based on its pleiotropy, the potential value of Rosuvastatin Calcium in areas such as heart failure, atrial fibrillation, postoperative complications of heart surgery, and non-alcoholic fatty liver disease is also being studied. For example, some small studies suggest that it may alleviate the pathological progression of heart failure by improving endothelial function and anti-inflammatory effects.
Recent research studies have revealed the efficacy of rosuvastatin in attenuating neuroinflammation, reducing the progression of Alzheimer's disease, providing protection against cerebral ischaemia and spinal cord injury as well as ameliorating epilepsy.
Related exploration: the "barometer" of cutting-edge research and future development
The research on Rosuvastatin Calcium has never stopped, and these explorations continue to deepen our understanding of it and guide the optimization direction of raw materials and formulation processes.
Genetic polymorphism and personalized medication: The coding gene SLCO1B1 of transporter OATP1B1 has polymorphism (such as alleles 5 and 15), which can lead to a decrease in its transport function. Patients carrying these alleles have reduced liver uptake of Rosuvastatin, increased plasma drug concentrations, and correspondingly increased risk of muscle toxicity. Therefore, genetic screening of high-risk populations to achieve dose individualization is an important practice in precision medicine. This in turn requires the production of active pharmaceutical ingredients to maintain a high level of inter batch consistency, ensuring the predictability of genotype phenotype relationships.
Green synthesis process development: The traditional Rosuvastatin Calcium synthesis route has longer steps and may use some environmentally unfriendly or expensive reagents. The current research focuses on developing green synthesis routes that are more efficient, atomically economical, and use safer solvents and catalysts. For example, optimizing asymmetric catalytic steps to improve the yield and optical purity of key chiral intermediates; Or develop a one pot continuous flow synthesis technology to improve production efficiency and safety. These technological innovations aim to reduce the production cost and environmental footprint of active pharmaceutical ingredients.
New delivery systems and compound formulations: Rosuvastatin Calcium is being developed into various new formulations to improve patient compliance. For example, it can be combined with Ezetimibe (a cholesterol absorption inhibitor) to form a fixed dose compound tablet, which synergistically lowers cholesterol levels through a dual mechanism. Or develop sustained-release formulations to achieve a dosing frequency of once a day or even once a week. This poses new requirements for the micronization technology of raw materials and their compatibility with excipients.
Deep comparison between biosimilars and original drugs: With the expiration of patents, Rosuvastatin Calcium's biosimilars (more accurately, chemical generic drugs) have been launched in large numbers. Regulatory agencies require generic drugs to undergo strict bioequivalence testing. However, subtle differences in crystal form, particle size, and other properties of the original powder may still affect the in vivo behavior of the formulation. In depth in vitro dissolution curve comparison, in vivo and in vitro correlation studies, and real-world big data research are continuously verifying the substitutability of high-quality generic drugs.
Comparison with other drugs: positioning in the "star map" of the statin family
Comparing statins to a family, Rosuvastatin Calcium is undoubtedly the "high-performance representative" among them. Through horizontal comparison, its unique value can be more highlighted.
Vs. Early liposoluble statins (Lovastatin, Simvastatin):
Potency strength: Rosuvastatin Calcium has the strongest potency in milligrams. To achieve the same level of LDL-C reduction, the required dose is minimal (milligrams vs. tens or even hundreds of milligrams).
Liver selectivity: Rosuvastatin Calcium has higher liver selectivity, relies on OATP1B1 transport, has less exposure to peripheral tissues, and theoretically has a lower risk of muscle toxicity. Fat soluble statins are distributed throughout the body through passive diffusion, and the mitochondrial function of muscle cells is more easily affected.
Metabolic pathway: Only about 10% of Rosuvastatin Calcium is metabolized by CYP2C9, and 90% is excreted in its original form. The risk of drug interactions is significantly lower than Lovastatin and Simvastatin, which are mainly metabolized by CYP3A4. This is particularly important for elderly patients who need to take multiple medications, such as anticoagulants and antiarrhythmic drugs.
Vs. Another potent lipophilic statin (Atorvastatin):
Lipid lowering efficacy: Within the conventional dosage range, both have comparable LDL-C lowering effects and are highly effective. But at the maximum dose, the decrease in Rosuvastatin Calcium 40 mg may be slightly better.
Duration of action: Atorvastatin and its active metabolites have a longer half-life (about 14 hours), while the half-life of Rosuvastatin Calcium prototype is about 19 hours. The inhibitory effect of Rosuvastatin Calcium on HMG CoA reductase may last longer because the enzyme's circadian rhythm is most active at night, and Rosuvastatin Calcium's half-life allows it to maintain an effective concentration at night.
The impact on HDL-C and TG: Both can moderately increase HDL-C and decrease triglycerides (TG). In some studies, the effect of Atorvastatin in reducing TG may be slightly significant.
Security spectrum: Both have overall good security. In large-scale clinical trials, the incidence of elevated liver enzymes (>3 times ULN) in both cases is very low (about 0.5% -1%). The incidence of muscle pain is also similar. However, Rosuvastatin Calcium has been reported to cause proteinuria (usually transient, benign, unrelated to glomerular filtration rate), which is less common in atorvastatin and may be related to tubular secretion.
Vs. other lipid-lowering drugs:
Ezetimibe: Mechanism complementarity. Ezetimibe inhibits intestinal cholesterol absorption, while Rosuvastatin Calcium inhibits liver synthesis. The combination of the two can produce a synergistic effect and is an important strategy for enhancing lipid-lowering. Compound preparations have improved compliance.
PCSK9 inhibitors: This new type of biological agent increases LDLR and reduces LDL-C by inhibiting PCSK9 protein, which is highly effective (reducing 50% -70%) and expensive. Rosuvastatin Calcium is an oral basal treatment, and PCSK9 inhibitors are commonly used in extremely high-risk patients who still do not meet the standard or cannot tolerate statins in combination with Ezetimibe.
Conclusion
The journey of Rosuvastatin Calcium, from a blueprint with complex molecular formulas to a pure white powder that has undergone countless trials and rigorous quality control, is a microcosm of the science and art of modern pharmaceutical industry. Its molecular structure embodies the wisdom of rational drug design, and its properties and characteristics explain the rigor of raw materials as the "soul" of formulations. Its principle of action reveals the ingenuity of precise intervention in diseases at the cellular level. As a cornerstone drug in the field of cardiovascular disease prevention and treatment, it not only reduces LDL-C levels worldwide, but also prevents countless myocardial infarction and stroke. With the deepening understanding of gene polymorphism and pleiotropy mechanisms, as well as the continuous development of green synthesis processes and new delivery systems, Rosuvastatin Calcium API powder and its formulations will continue to evolve and have a more profound impact in the fields of personalized medicine and public health. It is not just a chemical, but also a shining coordinate in the process of human use of scientific weapons to combat diseases.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality Rosuvastatin Calcium API powder, that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of Rosuvastatin Calcium , please contact our technical team through the following methods: sales12@faithfulbio.com.
Reference
1.McTaggart, F. (2003). Comparative pharmacology of rosuvastatin. Atherosclerosis Supplements, 4(1), 9-14.
2.Nissen, S. E., Nicholls, S. J., Sipahi, I., et al. (2006). Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA, 295(13), 1556-1565.
3.Ridker, P. M., Danielson, E., Fonseca, F. A., et al. (2008). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine, 359(21), 2195-2207.
4.Link, E., Parish, S., Armitage, J., et al. (2008). SLCO1B1 variants and statin-induced myopathy—a genomewide study. New England Journal of Medicine, 359(8), 789-799.
5. Chiang, Y., Kuo, C., & Wu, C. (2014). Analytical methods for determination of rosuvastatin calcium and related substances: a comprehensive review. Journal of Pharmaceutical and Biomedical Analysis, 88, 383-393.



