What exactly is Tianepine SodiumPowder?
What exactly is it? -Exploration of chemical essence and physical characteristics
In the vast field of neuroscience and pharmaceutical industry, there is a substance that attracts the attention of researchers and industry with its unique chemical structure, subverting the traditional pharmacological mechanism and complex industry ecology-Tianeptine sodium powder. It reflects the challenges, opportunities and profound insights of neuropsychiatric drug research and development from different angles. We will explore the whole picture of this special compound in depth.
To understand the position of Tianeptine Sodium Salt in the industry, we must first return to its essence: what kind of substance is it?
1. Chemical identity analysis:
The chemical name of Tianeptine Sodium Salt is 7-[(3-chloro-6,11-dihydro-6-methyldibenzo [c, f] [1,2] thiazepin-11-yl) amino] heptanoic acids, s-dioxide sodium salt. From its long name, we can interpret the key information: it is a derivative of tricyclic structure, but it is not a traditional tricyclic inhibitory product. Its molecular formula is C₂₁H₂₄ClN₂NaO₄S and its molecular weight is about 458.93 g/mol. The so-called "sodium salt" refers to the salt formed by the combination of the free base form of Tianeptine and sodium ions. This step is by no means a simple physical mixing, but a chemical modification aimed at significantly improving its key properties.
2. Physical characteristics and industrial significance:
It usually appears as white to white-like crystalline powder. This physical form is very important for storage, transportation, quality control and subsequent preparation production.
Solubility: Its greatest advantage is that it greatly improves water solubility. The free base form of Tianeptine is extremely poor in water solubility, which seriously limits its bioavailability and development. However, its solubility in water can usually exceed 100 mg/mL after being converted into sodium salt. This characteristic enables it to be conveniently used to prepare and develop various derivatives, which greatly expands its application scope.
3.Stability: Tianeptine sodium powder is sensitive to light, especially in the powder state. Long-term exposure to ultraviolet rays may trigger degradation reaction, leading to the decrease of purity and the generation of unknown impurities. Therefore, industry standards require the use of light-proof containers (such as brown glass bottles or aluminum foil bags) for packaging and storage. At the same time, it is also sensitive to temperature and humidity. The ideal storage condition is in a closed container, in a cool (usually recommended below 25°C) and dry environment. High temperature and high humidity will accelerate its hydrolysis or oxidation, affecting chemical stability and validity. These stability considerations directly determine its production technology, packaging materials, supply chain management and shelf life.
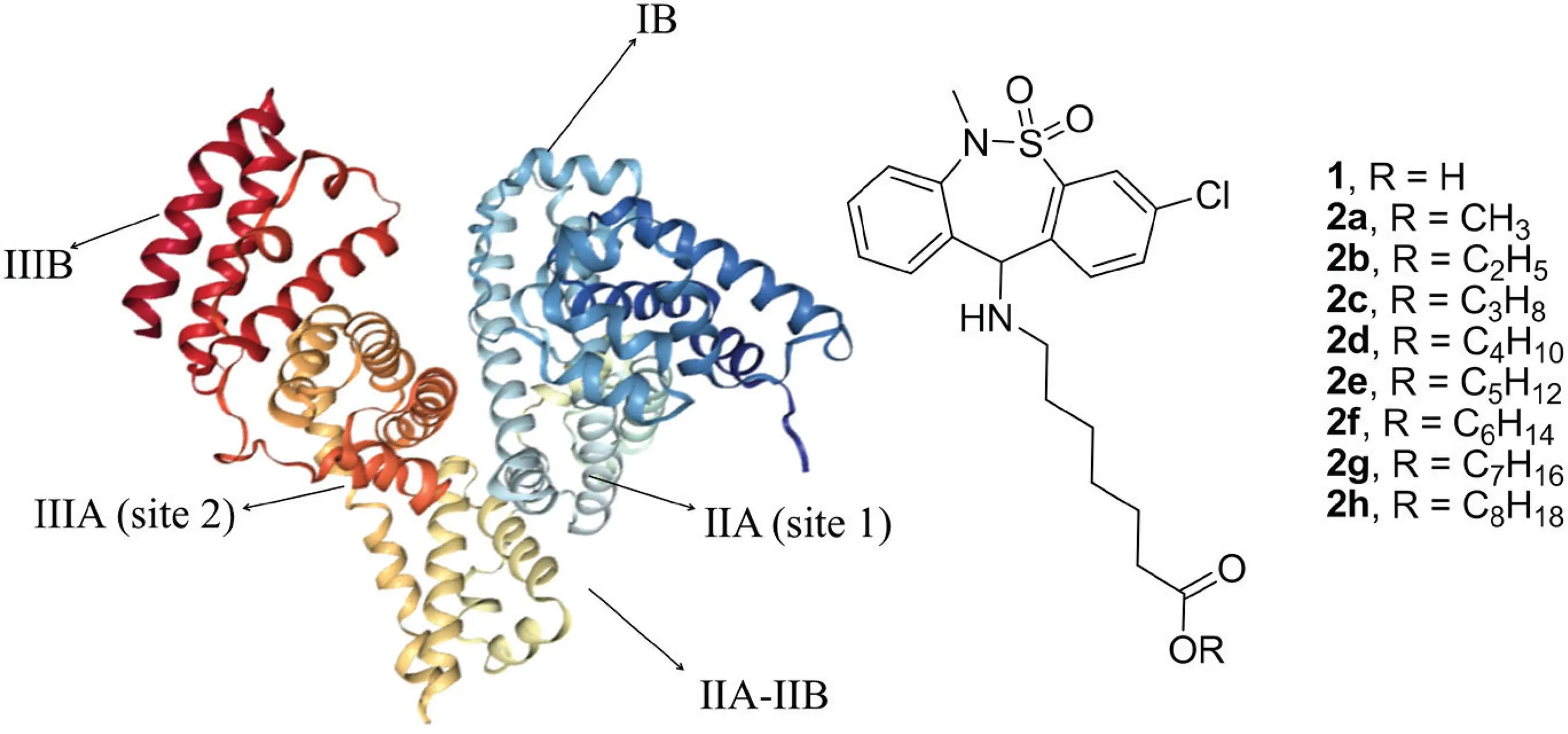
How does it work? -subverting the evolution of traditional pharmacological mechanisms
Its most fascinating place lies in the uniqueness of its pharmacological mechanism and the evolution of our understanding of it. How does it act on the brain to produce the observed effect?
1. Historical cognition: the paradox of serotonin enhancing reuptake agent
When it was first introduced, the scientific community generally believed that the mechanism of antidepressants was either to inhibit the reuptake of monoamine neurotransmitters or to antagonize their receptors. However, Tianeptine sodium powder shows a "paradox": early in vitro studies show that instead of inhibiting serotonin reuptake, Tianepine can enhance the function of serotonin transporter and accelerate the clearance of serotonin in synaptic cleft. This runs counter to the mainstream theory at that time. However, a large number of in vivo studies and clinical data have confirmed its clear anti-depression and anti-anxiety effects. This contradiction urges scientists to explore its deeper and more complex mechanism.
2. Modern paradigm: the core regulator of glutamatergic system and neuroplasticity
In the past twenty years, the research has gradually shifted the focus from a single monoamine system to the "chief excitatory transmitter" of the central nervous system-the glutamatergic system. At present, it is believed that its core mechanism may lie in its fine regulation of brain stress response and neuroplasticity:
Restorer of glutamate homeostasis: Chronic stress and depression are closely related to glutamate signal imbalance in specific areas of the brain, especially the hippocampus and prefrontal cortex, which are characterized by excitotoxicity and loss of synaptic connections. It is found that it can help to restore the steady state of glutamic acid. It may accelerate the clearance of excess glutamate from synaptic cleft by up-regulating the expression and function of glutamate transporter on astrocytes, thus reducing excitability.
Promoter of synaptic plasticity: synaptic plasticity is the basis of brain learning and adaptation, and its damage is one of the core pathologies of depression. It has been proved that it can reverse the dendritic atrophy and synaptic loss of hippocampal neurons caused by stress. It may enhance the long-term enhancement of synaptic transmission by affecting the function of receptors, which is the key mechanism of memory formation, thus promoting the recovery of neural plasticity.
Neuroprotection and neurotrophic effect: The study also shows that Tianeptine has a certain neuroprotective effect. It may increase the expression of brain-derived neurotrophic factor in some brain regions by inhibiting the negative effects of glucocorticoid induced by stress, thus supporting the survival and health of neurons.
This cognitive change from "monoamine paradox" to "glutamate/neuroplasticity regulator" not only explains its curative effect, but also puts Tianeptine sodium powder in a broader picture of neuropsychiatric treatment, laying a theoretical foundation for understanding its potential pleiotropic effects.

How was it born? -the challenges of synthetic technology and industrial manufacturing
From gram-scale synthesis in the laboratory to industrial mass production to meet market demand, the manufacturing of Tianeptine Sodium Salt is a road full of technical challenges.
1. Overview of synthetic routes:
The typical synthesis path is a multi-step organic synthesis process, and the key steps include:
Skeleton construction and functionalization: through classical organic reactions such as Ullmann reaction and coupling, the core tricyclic structure is constructed and modified, and key atoms and methyl groups are introduced.
Introduction of side chain: On the 11th carbon of the core skeleton, the side chain containing amino group is connected through nucleophilic substitution and other reactions. The stereoselectivity and reaction efficiency of this step are the keys to determine the total yield.
Salt formation and purification: finally, the obtained Tianeptine free base reacts with sodium carbonate in a suitable solvent to form Sodium salt. Then through fine chemical operations such as recrystallization, washing and drying, the high-purity final Tianeptine sodium powder is obtained.
2. The core challenges of industrial production:
Impurity spectrum control: A variety of process impurities will be produced in the synthesis process, such as unreacted intermediates, side reaction products and residual metal catalysts. Strict on-line process control and impurity mass spectrometry analysis of the final product (usually identified by high performance liquid chromatography (HPLC) combined with mass spectrometry (MS)) are the lifelines to ensure product safety and consistency. Industry standards have extremely strict upper limits on the contents of single unknown impurities and total impurities.
Crystal form control: it may have multiple crystal forms (polymorph phenomenon). Different crystal forms may have significant differences in solubility, stability, hygroscopicity and processability. Industrial production must be able to produce the target crystal form stably and repeatedly to ensure the consistency of final batch quality and expected in-vivo properties. This requires precise control of the crystallization process.
Green chemistry and sustainability: Modern API manufacturing pays more and more attention to environmental friendliness and sustainability. We choose greener synthetic routes, such as using green and safe solvents, developing reactions with higher atomic economy, and reducing the generation of waste water and waste residue. Our process optimization is not only related to cost, but also pays more attention to social responsibility and an important part of legal compliance.
How to ensure its quality? -Strict quality control system
For a highly active substance like Tianeptine Sodium Salt, the quality is not empty talk, but is defined and guaranteed by a set of strict standards and testing methods.
Quality standard framework:
Key quality attributes (CQAs) and detection methods;
Every batch of Tianeptine sodium powder must undergo a series of rigorous tests to ensure that it meets the preset CQAs:
Identification: "This is Tianeptine Sodium Salt" is confirmed by infrared spectrum (IR), ultraviolet spectrum (UV) or advanced mass spectrum (MS) and nuclear magnetic resonance spectrum (NMR) to prevent confusion.
Content determination: accurate determination of the content of the main component by HPLC and other precision instrument analysis methods. Pharmaceutical grade API usually requires a purity of not less than 98.5% or even 99.0%.
Related substances: This is the core of quality control. All known and unknown organic impurities are detected qualitatively and quantitatively by validated HPLC method to ensure that they are below the safety threshold.
Residual solvents: Organic solvents used in synthesis and purification must be effectively removed. Use gas chromatography to detect the residual amount of various solvents to ensure that it is below the prescribed exposure limit.
Physical and chemical properties: including loss on drying/moisture content, ignition residue (measuring inorganic impurities), etc.
Microbial limit: according to the use, it is necessary to control the total number of bacteria, yeast and mold, and detect specific pathogens. Sterility inspection and bacterial endotoxin test are needed.
This interlocking quality control system is a key bridge for us to ensure that Tianeptine sodium powder is transformed from an intermediate in a chemical plant into a high-standard substance for scientific research or medicine.
conclusion:
Tianeptine Sodium Salt powder, starting from its unique chemical structure, has experienced a cognitive revolution in its pharmacological mechanism from paradox to neuroplasticity regulator, from challenging synthesis process to extremely demanding quality control system, and finally serves a variety of application scenarios from basic scientific research to drug development. It is not only a chemical substance, but also a window, through which we can get a glimpse of the development of modern neuropharmacology, the precision and rigor of technology, and the continuous self-renewal of scientific cognition.
Despite the challenges, it represents a new treatment paradigm for complex brain diseases, which will continue to drive the scientific research and industrial practice around this unique compound to the future. A comprehensive understanding of its industry knowledge is very important for any researcher, engineer, analyst and decision maker who is in or concerned about this field.
Xi'an Faithful BioTech Co., Ltd. combines cutting-edge production technology with comprehensive quality assurance to provide high-quality Tianeptine sodium powder that meets international pharmaceutical standards. Our commitment to excellent, competitive prices and technical support makes us the preferred partner of global healthcare providers and researchers. Please contact our technical team in sales11@faithfulbio.com to find out how our products can improve your formula.
This is a list of the names of the core scientific research documents that I referred to and relied on in the process of writing a soft article. These documents provide solid scientific evidence for the efficacy and mechanism mentioned in this paper.
- Goodman & Gilman's The Pharmacological Basis of Therapeutics
- Naunyn–Schmiedeberg’s Archives of Pharmacology,2013
- Activation of μ receptors by SR-17018 through a distinctive mechanism
- The Predominant Protective Effect of Tianeptine Over Other Antidepressants in Models of Neuronal Apoptosis: The Effect Blocked by Inhibitors of MAPK/ERK1/2 and PI3-K/Akt Pathways
- Disposition of tianeptine and its active metabolite MC5 in healthy volunteers
- Pharmacokinetic study of tianeptine and its active metabolite MC5 in rats following different routes of administration using a novel LC–MS/MS method
- Tianeptine and its main metabolite in chronic renal failure and hemodialysis(Fundamental and Clinical Pharmacology,1990)
- Pharmacokinetics and bioequivalence of two sodium salt formulations of tianeptine in healthy male volunteers
- The effect of pH on polymorph formation of the pharmaceutically active compound tianeptine(International Journal of Pharmaceutics,2012)



