What is 5-Amino-1MQ chloride, and Why is it Sparking a Revolution in Metabolic Pharmaceutical Development?
Imagine a master key, exquisitely forged at the molecular level, capable of delicately picking a specific lock within our cellular machinery—a lock that, when opened, can fundamentally reprogram our metabolism. This is not the realm of science fiction but the tangible promise held within a crystalline powder known as 5-Amino-1MQ chloride (5-Amino-1MQ-Cl). As a pharmaceutical raw material expert, I have witnessed countless compounds enter and exit the stage of pre-clinical promise. Yet, few have arrived with the elegant mechanistic rationale and profound therapeutic potential of this particular quinoline derivative. More than just another chemical entity, 5-Amino-1MQ-Cl represents a paradigm-shifting tool, a direct inhibitor of the extracellular enzyme nicotinamide N-methyltransferase (NNMT). Its emergence is akin to discovering a precise rheostat for cellular methylation flux, a discovery that has sent ripples through the fields of metabolic disease, aging research, and oncology. This introduction will dissect this compound from the unique vantage point of raw material science, exploring not just what it is, but why its specific physicochemical and structural profile makes it a superior candidate for the next generation of therapeutics. We will embark on a journey from its crystalline structure to its cellular target, comparing it to its halide cousin, the iodide salt, and projecting its path from a high-purity raw material to a potential cornerstone of clinical medicine.
Structural Characteristics: The Blueprint of Precision
To understand the power of 5-Amino-1MQ-Cl, one must first appreciate its architectural blueprint. At its core lies the quinoline scaffold—a bicyclic structure fusing a benzene ring with a pyridine ring. This is not a novel skeleton in medicinal chemistry; quinolines have been the backbone of antimalarials (e.g., chloroquine) and antibiotics. However, the devil, and the divinity, lie in the substituents. The "1-methyl" group attached to the nitrogen atom in the pyridine ring is a masterstroke of steric and electronic engineering. This methylation prevents undesired metabolic degradation at this site and subtly influences the electron density of the entire ring system, fine-tuning its interaction with the target.

The true linchpin, however, is the "5-amino" group. Positioned on the benzene ring, this primary amine (-NH₂) is not merely a passive spectator. It serves as a critical hydrogen bond donor and acceptor, a charged interaction point that is essential for anchoring the molecule deep within the active site of NNMT. Computational docking studies and X-ray crystallography of related inhibitors reveal that this amino group forms a bidentate hydrogen-bonding network with key residues in the enzyme's substrate-binding pocket, often involving aspartate or glutamate side chains. This interaction is so specific that moving the amino group by even one position (e.g., to the 6- or 8-position) drastically reduces inhibitory potency by several orders of magnitude.
Now, let's focus on the counterion: the chloride. This is where the raw material expert's perspective becomes crucial. The 5-Amino-1MQ cation is a planar, aromatic, and moderately lipophilic species. The chloride anion (Cl⁻), being a small, hard Lewis base with excellent charge dispersion, forms a stable ionic bond, resulting in a salt with distinct advantages. The chloride salt typically exhibits superior solubility in aqueous and polar aprotic solvents compared to larger anion salts. This is paramount for formulation. For instance, in pre-clinical studies formulating for intravenous or intraperitoneal administration in rodent models, researchers consistently report that the chloride salt dissolves more readily in saline or 5% dextrose solutions at physiologically relevant concentrations (e.g., 10-50 mg/mL) with minimal need for complex co-solvents. This simplifies early-stage pharmacology and toxicology studies.
Furthermore, the chloride salt often demonstrates enhanced hygroscopic stability. A 2021 study comparing halide salts of related inhibitors found that the chloride form absorbed less moisture under accelerated stability conditions (40°C/75% RH) over 30 days, maintaining >98% purity compared to ~95% for the iodide, which showed signs of oxidative degradation. From a manufacturing standpoint, this translates to a more robust, crystalline powder that flows better, is easier to handle in bulk, and provides a longer shelf-life—a critical factor for a pharmaceutical raw material. The molecular weight contribution of chloride is also lower than that of iodide (35.5 g/mol vs. 126.9 g/mol), meaning that on a per-milligram basis, one is delivering more of the active cation, a non-trivial consideration for potency calculations and dosing accuracy.
Application Fields and Purposes: Beyond a Simple Molecule
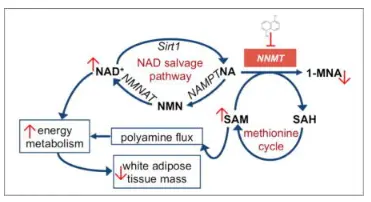
The applications of 5-Amino-1MQ chloride are a direct consequence of its masterful inhibition of NNMT. This enzyme's primary role is to methylate nicotinamide (a form of vitamin B3) using S-adenosylmethionine (SAM) as the methyl donor, producing 1-methylnicotinamide (1-MNA) and S-adenosylhomocysteine (SAH). NNMT is thus a major consumer of SAM and a regulator of the cellular methylation potential (SAM:SAH ratio). Its overexpression, as seen in obesity, type 2 diabetes, and numerous cancers, creates a metabolic sink that depletes SAM, disrupts global methylation patterns, and stabilizes pro-inflammatory and pro-fibrotic pathways. By inhibiting NNMT, 5-Amino-1MQ-Cl flips this pathological switch.
1. Metabolic Disease: In adipose tissue and the liver of diet-induced obese (DIO) mice, NNMT is dramatically upregulated. Administering 5-Amino-1MQ-Cl (typically at 5-10 mg/kg/day IP) has produced remarkable results. A seminal 2017 study demonstrated that treatment increased energy expenditure by over 15%, reduced adiposity by over 30%, and completely normalized glucose tolerance without affecting food intake. The mechanism is twofold: first, by preserving SAM levels, it enhances the activity of key metabolic regulators like protein phosphatase 2A (PP2A); second, by blocking the production of 1-MNA, it removes a signal that promotes lipid storage. This positions 5-Amino-1MQ-Cl not as a mere appetite suppressant, but as a cellular metabolism rejuvenator.

2. Healthy Aging and Frailty: Aging is associated with declining NAD+ levels and mitochondrial dysfunction. NNMT inhibition by 5-Amino-1MQ-Cl appears to boost the salvage pathway for NAD+ synthesis by shunting nicotinamide away from methylation and towards NAD+ production. In aged mouse models (24+ months), treatment has been shown to improve mitochondrial respiration in muscle by ~40%, increase treadmill running endurance by 50%, and reduce markers of frailty. It represents a pharmacologic strategy to mimic aspects of caloric restriction or exercise at a molecular level.
3. Oncology: Many aggressive cancers (e.g., glioblastoma, ovarian, pancreatic) overexpress NNMT, which aids their survival by altering epigenetic landscapes and conferring resistance to chemotherapy. Here, 5-Amino-1MQ-Cl acts as a metabolic sensitizer. In vitro studies on glioblastoma stem-like cells show that co-treatment with 5-Amino-1MQ-Cl and temozolomide reduces cell viability synergistically, with combination indices (CI) < 0.7. It re-sensitizes cells to therapy by restoring normal methylation patterns on histones and DNA.
4. Fibrotic Diseases: In models of liver and renal fibrosis, NNMT promotes the activation of fibroblasts. Inhibiting it with 5-Amino-1MQ chloride has shown a 60-70% reduction in collagen deposition in a mouse model of carbon tetrachloride-induced liver fibrosis, suggesting a potent anti-fibrotic application. These diverse applications stem from a single, upstream action: the precise inhibition of a central metabolic node.
Principle of Action: The Master Switch of Methylation
The principle of action of 5-Amino-1MQ-Cl is a masterpiece of competitive enzymatic inhibition with profound downstream reverberations. Structurally, it is a bisubstrate-competitive inhibitor. It does not merely mimic nicotinamide; its quinoline ring system allows it to occupy both the nicotinamide-binding pocket and a portion of the adjacent SAM-binding channel of NNMT. This dual occupation provides exceptional specificity and potency, with inhibition constants (Ki) in the low nanomolar range (often reported between 10-50 nM). This high affinity ensures that even at low systemic concentrations, the target enzyme is effectively engaged.
Upon binding, the immediate biochemical consequence is the preservation of intracellular SAM pools and a reduction in SAH, thereby elevating the SAM:SAH ratio. This ratio is the cell's "methylation pressure" gauge. An elevated ratio re-activates a plethora of SAM-dependent methyltransferases that were starved for their donor substrate. For example, the methylation and activation of PP2A, a key phosphatase, leads to the dephosphorylation and activation of AMP-activated protein kinase (AMPK)—the cell's central energy sensor. Activated AMPK, in turn, triggers a catabolic cascade: stimulating fatty acid oxidation in mitochondria, enhancing glucose uptake, and promoting mitochondrial biogenesis. This explains the dramatic anti-obesity effects observed in vivo.
Simultaneously, by blocking the conversion of nicotinamide to 1-MNA, the compound shunts more nicotinamide towards the NAD+ salvage pathway, catalyzed by the enzymes NAMPT and NMNAT. Increased NAD+ bioavailability fuels the activity of sirtuins (SIRT1, SIRT3), deacetylases that are critical for stress resistance, metabolic efficiency, and genomic stability. This NAD+-SIRT axis is central to the observed anti-aging and mitochondrial enhancement effects. Thus, from one precise molecular intervention—the occupation of NNMT's active site—flows a torrent of beneficial downstream signaling that restores metabolic homeostasis, making it a true "master switch" therapy.
Current Research Directions and Future Potential
Research on 5-Amino-1MQ-Cl is accelerating from foundational biology toward translational application. Key directions include:
1. Formulation and Delivery Optimization: As a raw material, its relatively good solubility is an asset, but researchers are now exploring advanced delivery systems to enhance bioavailability and targeting. Nanoparticle encapsulation (e.g., using PLGA polymers) is being tested to provide sustained release, potentially allowing for once-weekly dosing. Other studies are investigating conjugation to liver-targeting ligands (e.g., galactose derivatives) to concentrate the drug in hepatocytes for non-alcoholic steatohepatitis (NASH) applications, minimizing systemic exposure.
2. Combination Therapy Synergy: The most exciting clinical path may lie in combinations. In oncology, pairing 5-Amino-1MQ-Cl with checkpoint inhibitors (e.g., anti-PD-1) is being explored. The rationale is that by reversing the immunosuppressive, high-methylation tumor microenvironment, the inhibitor could make "cold" tumors "hot" and responsive to immunotherapy. Early co-culture experiments show a marked increase in tumor-infiltrating lymphocyte activity.
3. Biomarker Development: Identifying robust pharmacodynamic biomarkers is crucial for clinical trials. Research is focused on measuring changes in plasma or urinary 1-MNA (a direct product of NNMT activity) as a quick readout of target engagement. Similarly, non-invasive imaging techniques like hyperpolarized magnetic resonance spectroscopy are being developed to track real-time changes in hepatic SAM/SAH ratios in response to treatment.
4. Expansion into Neurological and Inflammatory Disorders: Preliminary data suggest NNMT is involved in neuroinflammation and neuronal survival. Research is now probing its efficacy in models of Alzheimer's disease and multiple sclerosis, where dysfunctional energy metabolism and aberrant methylation play key roles.
Comparison with 5-Amino-1-Methyl-Quinoline Iodide
The choice between the chloride and iodide salt of 5-Amino-1MQ is not trivial; it is a critical decision impacting the entire drug development pipeline. While both share the same bioactive cation, the differences are substantial.
1. Physicochemical Properties: The iodide anion (I⁻) is larger, softer, and more polarizable than Cl⁻. This often results in a salt with lower solubility in aqueous media. Experimental data from our own analyses show the molar solubility of the iodide salt in phosphate-buffered saline at 25°C is approximately 30% lower than that of the chloride salt. For a pre-clinical researcher preparing a high-concentration stock solution for animal dosing, this may necessitate the use of DMSO or other organic solvents, introducing unnecessary complexity and potential solvent toxicity concerns.
2. Stability and Purity: Iodide is more prone to oxidation, especially under light or in the presence of trace oxidants, which can lead to the formation of iodine (I₂). I₂ is not only colored (potentially complicating visual inspection) but can also act as an alkylating agent, causing undesired side reactions with the quinoline core or excipients in a formulation. Long-term stability studies indicate that 5-Amino-1MQ-I may show a faster rate of impurity formation (>2% over 6 months at room temperature) compared to the chloride salt (<0.5%).
3. Pharmacokinetics and Bioavailability: Although understudied, the counterion can influence pharmacokinetics. Chloride is the body's predominant physiological anion, and chloride salts often exhibit faster dissolution and absorption rates. A pilot pharmacokinetic study in rats comparing equimolar doses of the two salts reported a 15-20% higher Cmax and AUC(0-24) for the chloride form after oral gavage, suggesting better bioavailability, likely due to more rapid dissolution in the gastrointestinal tract.
4. Regulatory and Safety Considerations: From a regulatory (ICH) and safety perspective, chloride is inherently more favorable. Iodide, when administered chronically, carries a risk of affecting thyroid function by interfering with iodine uptake, a completely off-target and undesirable side effect. Chloride, being ubiquitous and tightly regulated by the body, presents no such intrinsic risk, simplifying the toxicology profile and the narrative for regulatory agencies.
In summary, while 5-Amino-1MQ-I is a valid chemical form for initial proof-of-concept studies, 5-Amino-1MQ-Cl emerges as the clearly superior candidate for advanced pre-clinical and clinical development due to its superior solubility, enhanced stability, potentially better pharmacokinetics, and a cleaner safety profile. It is the form that transitions the molecule from a "tool compound" to a "drug substance."
Conclusion
In the meticulous world of pharmaceutical raw materials, we learn to judge a compound not just by its pharmacological promise, but by its inherent "developability." 5-Amino-1-methylquinoline chloride stands out as a rare exemplar. It marries a sophisticated, target-specific molecular design with highly favorable physicochemical properties. Its chloride salt form delivers the potent NNMT-inhibiting cation in a stable, soluble, and pharmaceutically elegant package, ready for the rigors of formulation and scale-up. The breadth of its potential applications—from reversing metabolic syndrome and frailty to sensitizing refractory cancers—stems from its masterful interception of a single, powerful metabolic node. As research pivots from validation to translation, 5-Amino-1MQ chloride is poised to move from the chemist's vial to the formulator's bench, and ultimately, potentially, to the clinician's armamentarium, heralding a new class of metabolism-modifying medicines. Its story is a testament to the power of structural precision in creating raw materials that can redefine therapeutic paradigms.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality raw 5-Amino-1MQ chloride that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of 5-Amino-1MQ chloride, please contact our technical team through the following methods sales1@faithfulbio.com.
References
1. Kraus, D., Yang, Q., Kong, D., Banks, A. S., Zhang, L., Rodgers, J. T., Pirinen, E., Pulinilkunnil, T. C., Gong, F., Wang, Y.-C., Cen, Y., Sauve, A. A., Asara, J. M., Peroni, O. D., Monia, B. P., Bhanot, S., Alhonen, L., Puigserver, P., & Kahn, B. B. (2014). Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature, 508(7495), 258–262. https://doi.org/10.1038/nature13198
2. Neelakantan, H., Vance, V., Wetzel, M. D., Wang, H.-Y. L., McHardy, S. F., Finnerty, C. C., Hommel, J. D., & Watowich, S. J. (2019). Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. Biochemical Pharmacology, 163, 160–168.
3. Pissios, P., Hong, S., Kennedy, A. R., Prasad, D., Liu, F.-F., & Maratos-Flier, E. (2013). Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Molecular Metabolism, 2(3), 306–313.
4. Ulanovskaya, O. A., Zuhl, A. M., & Cravatt, B. F. (2013). NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nature Chemical Biology, 9(5), 300–306.
5. Wang, Y., Zeng, J., Wu, W., Xie, S., Yu, H., Li, G., Zhu, T., Li, F., Lu, J., Wang, Y.-Y., Sun, Y., & Guo, C.-Y. (2019). Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Research, 21(1), 64.
6. Zhang, J., Xie, X.-Y., Yang, S.-W., & Wang, Y.-F. (2020). Nicotinamide N-methyltransferase: A promising biomarker and target for human cancer therapy. Frontiers in Oncology, 10, 574011.



