What is Dynorphin A (1-13) peptide?
What is Dynorphin A (1-13)?
Dynorphin A (1-13) is an endogenous neuropeptide. This name sounds a little complicated, but it is actually a biologically active substance naturally produced by our body, which plays an important regulatory role in the nervous system.
From the chemical structure, Dynorphin A (1-13) peptide consists of 13 amino acids, and its sequence is Tyr-Gly-Gly-Phe-Leu-Arg-Arg-ile-Arg-Pro-Lys-Leu-Lys. This sequence is abbreviated as YGGFLRRIRPKLK in single letter notation. Its molecular formula is C75H126N24O15 and its molecular weight is about 1603.97. This polypeptide mainly exists in central nervous system and peripheral organs, especially in striatum and substantia nigra.
The Discovery History of Dynorphin A (1-13)
The discovery of Dynorphin A (1-13) can be traced back to 1979, when scientists successfully isolated it from pig pituitary for the first time. This discovery marks an important milestone in the field of peptide research. Scientists have found that this endogenous substance can combine with other receptors and play a role, but it is naturally produced by our body, so it is called "endogenous peptide".
Subsequent studies gradually revealed the complete amino acid sequence and biological function of Dynorphin A (1-13). In 2023, the team of Shanghai Institute of Pharmacology, Chinese Academy of Sciences successfully analyzed the three-dimensional structure of it and its complex by cryoelectron microscopy. This breakthrough research provided detailed information at the molecular level for understanding its mechanism of action.
The mechanism of Dynorphin A (1-13)
1. Receptor binding characteristics
Dynorphin A (1-13) peptide is a potent agonist. This means that it can specifically bind to it and activate these receptors. This combination has high affinity and selectivity, which makes it play a significant biological effect at physiological concentration.
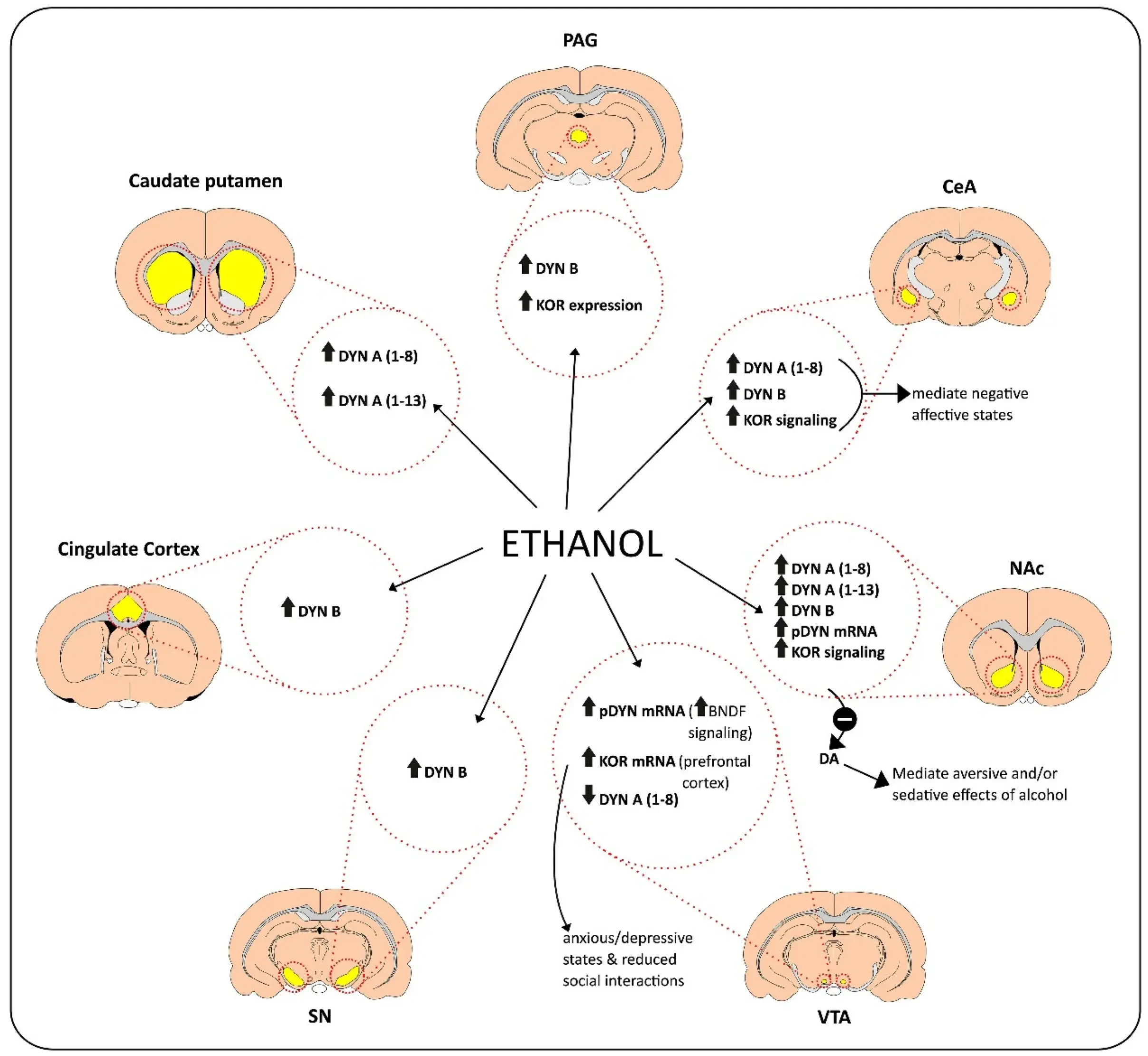
It was found that the binding of Dynorphin A (1-13) to the receptor was realized through its N-terminal Tyr-Gly-Gly-Phe-Leu sequence, which constituted the key domain for binding to the receptor. However, many positively charged amino acid residues in the sequence complement the negative region of the receptor through electrostatic interaction, thus achieving selective activation.
2. Signal transduction pathway
When Dynorphin A (1-13) binds to the receptor, it will induce the conformational change of the receptor, and then activate the downstream signal transduction pathway. Specifically, the activated receptor can be coupled with G protein, which inhibits the activity of Adenylate cyclase and reduces the production of intracellular cAMP. CAMP is an important second messenger in cells, and the decrease of its concentration will further affect a series of cell functions.
In addition, it may also regulate the excitability of neurons by affecting the activity of ion channels. For example, it can inhibit the influx of calcium ions and promote the outflow of other ions, thus reducing the excitability of neurons. This regulation mechanism is very important for physiological processes such as pain perception and emotional regulation.
3. Physiological concentration and toxicity
At physiological concentration, it mainly plays the role of analgesia and neuromodulation. However, when the concentration is too high, Dynorphin A (1-13) peptide may have toxic effects on neurons. It is found that continuous exposure to 100 μM Dynorphin A (1-13) will lead to a significant decrease in neurons over time. This toxic effect may be related to the sharp increase of intracellular calcium concentration, which is similar to the effect seen by acute NMDA receptor activation.
Physiological functions of Dynorphin A (1-13)
1. Analgesic effect
The most remarkable function of Dynorphin A (1-13) is its analgesic effect. As an endogenous ligand, it can relieve pain by inhibiting the release of neurotransmitters. This analgesic effect is effective for many types of pain, including acute pain, chronic pain and neuropathic pain. It is worth noting that, compared with traditional receptor agonists, it is often accompanied by lower risk of addiction, less respiratory depression and constipation while relieving pain.
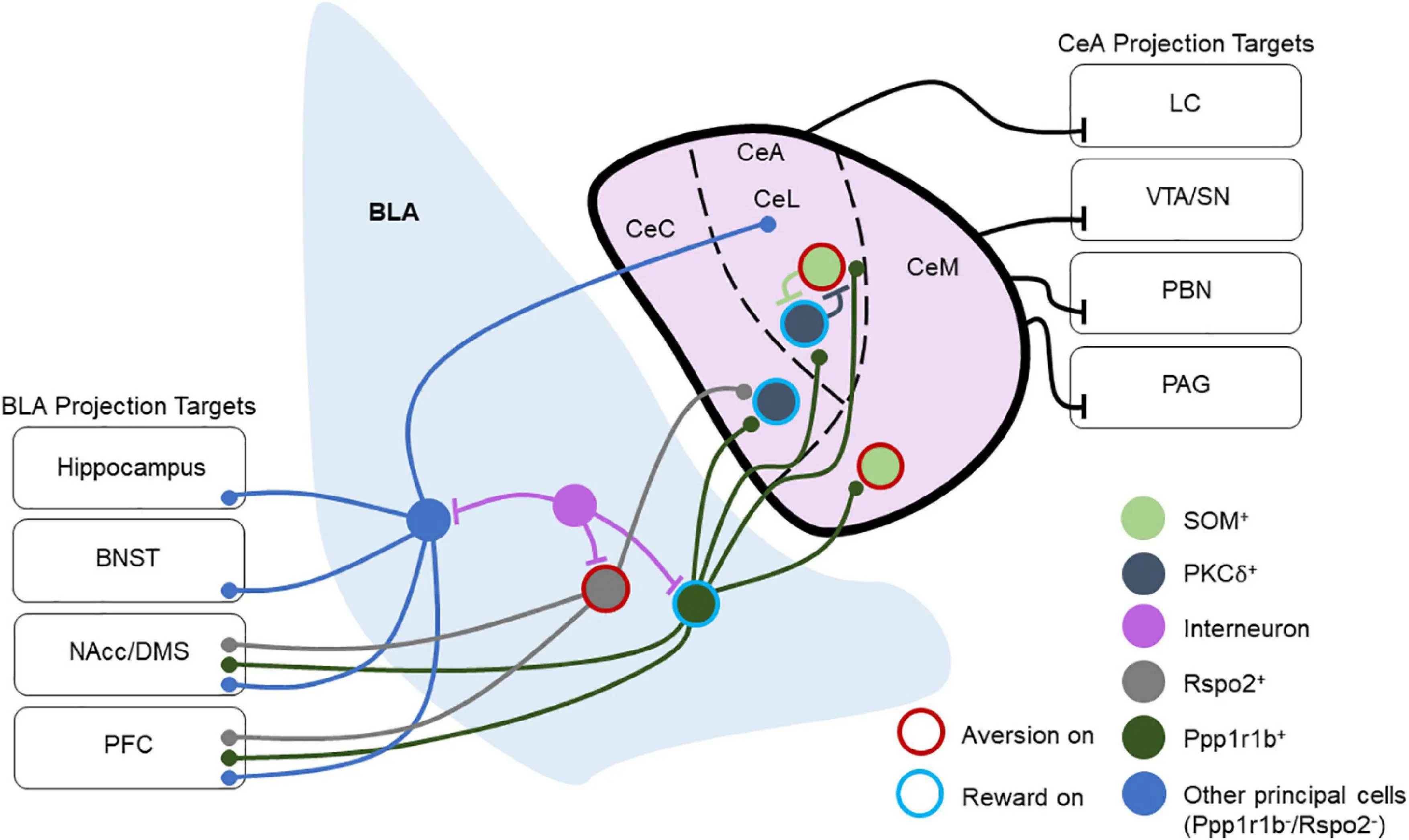
2. Emotional adjustment
Dynorphin A (1-13) peptide is involved in the process of emotional regulation in the central nervous system. It is distributed in striatum, hippocampus and other brain regions, and affects reward mechanism and addiction by regulating dopaminergic pathway. Studies have shown that it may participate in the regulation of physiological functions such as anti-anxiety and anti-depression.
3. Cardiovascular regulation
Besides the function of nervous system, it also participates in the regulation of cardiovascular system. Myocardial ischemic preconditioning can induce the release of Dynorphin A and enhance the tolerance of the heart to ischemia-reperfusion injury. This effect can be simulated by receptor agonists or blocked by antagonists.
4. Neuroprotection and neurotoxicity
Its role in the nervous system is dual. At physiological concentration, it may have neuroprotective effect; But at high concentration, it may have toxic effects on neurons, leading to neuron death and morphological destruction. This dual effect makes its mechanism in nervous system diseases complicated.
Synthesis and production of Dynorphin A (1-13)
1. Chemical synthesis method
It can be synthesized. At present, the commonly used synthesis methods include solid-phase synthesis and liquid-phase synthesis. Solid-state synthesis is the mainstream method of polypeptide synthesis at present, and its advantages are simple operation, high yield and easy purification. Liquid phase synthesis is suitable for the synthesis of some special sequences of peptides.
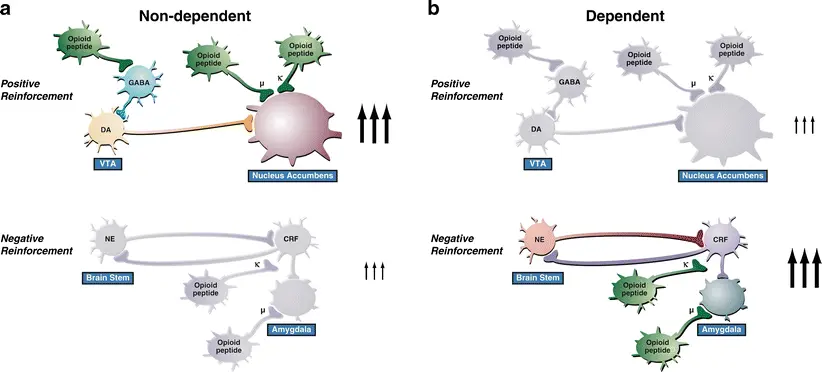
In the process of synthesis, corresponding amino acids need to be selected according to the amino acid sequence of Dynorphin A (1-13). Firstly, it is necessary to activate amino acids to make them more reactive, and then connect them one by one through condensation reaction to form peptide bonds. This process requires precise control of reaction conditions to ensure that the synthesized polypeptide has the correct sequence and structure.
2. Quality control
The synthesized Dynorphin A (1-13) peptide needs strict quality control. Commonly used quality control indicators include purity, molecular weight, amino acid composition analysis, secondary structure analysis and so on. The purity is usually above 98%, the molecular weight should be consistent with the theoretical value, and the amino acid composition should be consistent with the expected sequence.
3. Storage and stability
As a bioactive molecule, it needs to be preserved under appropriate conditions, and it is usually recommended to store it at -20℃ or lower to avoid repeated freezing and thawing. In solution, its stability may be affected by pH, temperature, ionic strength and other factors.
Research and application of Dynorphin A (1-13)
1. Neuroscience research
It is widely used in the field of neuroscience. By studying its mechanism of action in the nervous system, scientists can deeply understand the function and disease mechanism of the nervous system. These studies are helpful to reveal the molecular basis of complex neural processes such as pain perception, emotional regulation, learning and memory.
2. Pharmacological research
In the field of pharmacology, it is an important tool molecule for studying receptors. By studying its binding characteristics with receptors, signal transduction mechanism and physiological effects, it can provide an important theoretical basis for developing new analgesic products.
3. Drug development
Based on its structure and mechanism of action, scientists are developing new analgesic drugs. These drugs aim to reduce addiction and other side effects while retaining their analgesic effects. Structural modification strategies include targeted modification and stability enhancement modification on the basis of retaining the core sequence.
The future research direction of Dynorphin A (1-13)
1. Structural biology research
With the development of structural biology techniques such as cryoelectron microscopy, the complex structure of Dynorphin A (1-13) peptide and its receptor can be further analyzed in the future. These studies will reveal the precise molecular mechanism of binding and activating receptors, and provide structural basis for drug design.
2. Product development strategy
Based on the unique structure of Dynorphin A (1-13), a variety of product development strategies can be developed in the future. For example, develop pH-sensitive nano-preparation to trigger substance release in acidic environment of inflammatory tissue; Develop long-acting sustained-release preparations to achieve sustained release.
conclusion
As an endogenous neuropeptide, it plays an important role in pain regulation, emotional regulation and cardiovascular regulation. Its unique structural characteristics and mechanism of action make it an important tool molecule in neuroscience and pharmacology research, and also provide an important target framework for developing new analgesic products.
In the future, we can make better use of the endogenous substance Dynorphin A (1-13) and bring better quality of life to patients by continuously optimizing the synthesis process and deeply studying the mechanism of action. The research in this field not only has important scientific significance, but also has broad application prospects.
Xi'an Faithful BioTech Co., Ltd. combines cutting-edge production technology with comprehensive quality assurance to provide high-quality Dynorphin A (1-13) peptide that meets international pharmaceutical standards. Our commitment to excellent, competitive prices and technical support makes us the preferred partner of global healthcare providers and researchers. Please contact our technical team in sales11@faithfulbio.com to find out how our products can improve your formula.
This is a list of the names of the core scientific research documents that I referred to and relied on in the process of writing a soft article. These documents provide solid scientific evidence for the efficacy and mechanism mentioned in this paper.
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. (1979) Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci USA76(12):6666-6670
- Cox BM, Opheim KE, Teschemacher H, Goldstein A. (1975) Isolation from pituitary of a new peptide with opioid activity. Life Sci16(11):1777-1782
- Ferré G, Czaplicki G, Demange P, Milon A. (2019) Structure and dynamics of dynorphin peptide and its receptor. In: Vitamins & Hormones(Vol. 110, pp. 25-56). Academic Press
- Chavkin C, Goldstein A. (1981) Specific receptor for the opioid peptide dynorphin: structure-activity relationships. Proc Natl Acad Sci USA78(10):6543-6547
- Hauser KF, et al. (1999) Dynorphin A (1-13) neurotoxicity in vitro: opioid and non-opioid mechanisms in mouse spinal cord neurons. Exp Neurol160(2):361-375
- Naqvi T, Haq W, Mathur KB. (1998) Structure-activity relationship studies of dynorphin A and related peptides. Eur J Med Chem33(11):873-891
- ulunay FC, et al. (1981) The effect of dynorphin on morphine and beta-endorphin-induced analgesia. J Pharmacol Exp Ther219(2):296-298
- Mamiya T, et al. (2014) Dynorphin A (1-13) alleviated stress-induced behavioral impairments in mice. Biol Pharm Bull37(8):1269-1273
- Wang Q, et al. (2012) Endogenous dynorphin protects against neurotoxin-elicited nigrostriatal dopaminergic neuron damage and motor deficits in mice. J Neuroinflammation9:124
- Greenwald MK, Stitzer ML, Haberny KA. (1999) Human Pharmacology of the Opioid Neuropeptide Dynorphin A(1–13). J Pharmacol Exp Ther290(2):762-770
- King AC, Ho A, Borg L, Kreek MJ. (1999) Acute subjective effects of dynorphin A(1-13) infusion in normal healthy subjects. Psychopharmacology142(2):147-155
- Turcotte A, Lalonde JM, St-Pierre S, Lemaire S. (1989) Dynorphin-(1-13). I. Structure-function relationships of Ala-containing analogs. Int J Pept Protein Res33(4):361-367
- Brugos B, et al. (2004) Metabolism of dynorphin A(1-13). Pharmazie59(11):847-851
- Clavin W. (2005) Dynorphin: the natural antidote to cocaine (and pleasure). J Addict Med2(3):113-119
- Nyberg F, Hallburg M. (2007) Neuropeptides in the regulation of stress. Prog Brain Res170:47-62



