What is the anticancer effect of Abiraterone acetate powder?
Understanding Abiraterone acetate powder: from molecular structure to medicinal value
1. Chemical and physical properties
It is a white to white crystalline powder, and its molecular structure has been carefully designed, which not only ensures the stability of the product, but also ensures the effective transformation in the body. At room temperature, the properties of the powder are relatively stable, but Abiraterone acetate powder needs to be sealed and stored away from light to avoid moisture or high temperature environment.
2. Pharmacological mechanism
Abiraterone acetate specifically inhibits cytochrome P450c17(CYP17) enzyme, thus blocking the synthesis of androgen in testis, adrenal gland and prostate cancer tissues. This mechanism is aimed at the "fuel supply" of prostate cancer cells and fundamentally inhibits tumor growth. Different from traditional chemotherapy drugs, it belongs to the category of targeted therapy and has the characteristics of clear mechanism of action and relatively controllable side effects.
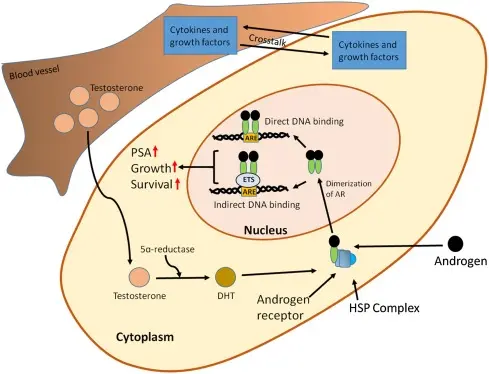
The Precise Strike of Abiraterone acetate powder —— "Cut off all fuel lines"
The role of Abiraterone acetate is to destroy the backup plan of cancer cells. The process is as follows:
Prodrug: It is a kind of "prodrug" and has no activity.
In vivo activation: after entering the human body, it releases the active ingredient-abiraterone through rapid water interpretation of esterase in the liver and blood.
Precise inhibition: active Abiraterone can effectively and selectively inhibit CYP17A1 enzyme. This enzyme is a "key valve" in androgen synthesis pathway, which controls two important pathways.
Total "fasting": by inhibiting CYP17A1 enzyme, Abiraterone acetate cuts off the source of adrenal gland and prevents the adrenal gland from producing androgen precursors. It also stops the self-supply of cancer cells and prevents cancer cells from synthesizing androgen by themselves in the tumor microenvironment.
In this way, whether cancer cells rely on external supply or internal production, their "fuel" supply is completely cut off. Without the stimulation of androgen, the growth of cancer cells will be significantly inhibited and even apoptosis.
Its anticancer effect can be brilliantly summarized as follows: Abiraterone acetate powder is not a "bomb" that directly kills cancer cells, but a brilliant "strategist". By precisely inhibiting CYP17A1, a key enzyme, it completely cuts off the "food and grass supply" (androgen) of prostate cancer cells, thus effectively inhibiting tumor growth and providing an important treatment choice for patients with advanced prostate cancer. However, its use must be carried out under the strict guidance and supervision of doctors.
From APIs to pharmaceutical products
1. The core position in the pharmaceutical industry
In the formal pharmaceutical field, it is mainly used for the production of raw materials. The production of APIs needs to meet the strict good manufacturing practice, so as to ensure the quality of the whole process from raw material procurement to finished product delivery.
2. Clinical application guidelines
According to the approval of National Medical Products Administration, Abiraterone acetate powder is suitable for: metastatic castration-resistant prostate cancer; Newly diagnosed high-risk metastatic endocrine-sensitive prostate cancer; Clinical use should strictly follow the doctor's advice, usually combined with Meprednisone to reduce the risk of mineralocorticoid-related side effects.

Production technology and quality control: high-tech manufacturing process
1. Key points of synthesis process
The synthesis of Abiraterone acetate is a complex multi-step reaction process, involving key technologies such as chiral synthesis and purification. Excellent production process needs to ensure: high selectivity of chemical reaction, high purity of intermediate and stability of final product.
At present, we have the production technology of leading enterprises in the industry, which has been able to achieve stable production on a kilogram scale.
2. Strict requirements of quality standards
Pharmaceutical grade Abiraterone acetate powder must meet the pharmacopoeia standards of various countries, including: the content is not less than 98.5%; Strictly control the content of related substances; Heavy metal residue limit; Microbial limit test; These standards ensure the safety and effectiveness of the final preparation products.
Conclusion:
With the acceleration of population aging and the improvement of health awareness, its market demand has been growing continuously. As an important anti-cancer raw material, its standardized production and rational use are directly related to the life and health of patients. We have been committed to providing a quality-assured supply chain of high-quality products: strictly control the quality of each batch and strengthen supervision in circulation. Contribute to the construction of human health.
Xi'an Faithful BioTech Co., Ltd. combines cutting-edge production technology with comprehensive quality assurance to provide high-quality Abiraterone acetate powder that meets international pharmaceutical standards. Our commitment to excellent, competitive prices and technical support makes us the preferred partner of global healthcare providers and researchers. Please contact our technical team in sales11@faithfulbio.com to find out how our products can improve your formula.
This is a list of the names of the core scientific research documents that I referred to and relied on in the process of writing a soft article. These documents provide solid scientific evidence for the efficacy and mechanism mentioned in this paper.
de Bono, J. S., et al. "Abiraterone and increased survival in metastatic prostate cancer." New England Journal of Medicine(2011).
Ryan, C. J., et al. "Abiraterone in metastatic prostate cancer without previous chemotherapy." New England Journal of Medicine(2013).
Attard, G., et al. "The development of abiraterone acetate for the treatment of advanced prostate cancer." Nature Reviews Clinical Oncology(2012).
de Bono, J. S., Logothetis, C. J., Molina, A., et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364(21): 1995-2005.
The United States Pharmacopeia, 43rd Revision. Abiraterone Acetate Monograph. Rockville, MD: USP Convention; 2020.
Attard, G., Reid, A. H., & de Bono, J. S. The development of abiraterone acetate for the treatment of advanced prostate cancer. Nat Rev Clin Oncol. 2012; 9(6): 325-330.
IQVIA Institute. Global Oncology Trends 2023. Parsippany, NJ: IQVIA; 2023.
European Medicines Agency. Assessment Report for Zytiga. EMA/CHMP/603088/2011. 2011.



