What is Vinpocetine Powder?
Know the basic information of Vinpocetine Powder
Vinpocetine is a high-purity powder used as a raw material for scientific research and medicine. Vinpocetine Powder belongs to Catharanthus roseus alkaloid derivative chemically. The common alias is Ethyl apovincaminate, with the logo of CAS: 42971-09-5, molecular formula: C22H26N2O2, and molecular weight: 350.45. The appearance is mostly white or white-like crystalline powder, which is almost insoluble in water; Its research orientation is voltage-gated sodium channel blockers and PDE inhibitors, which are used to improve cerebral microcirculation, inhibit inflammation and protect nerves in the related research of cerebrovascular and neuroprotection. The supply specification is usually ≥ 98% ~ 99%, and the powder is usually recommended to be kept dry and sealed at -20℃ in the dark.
How it works: mechanism and application scenarios
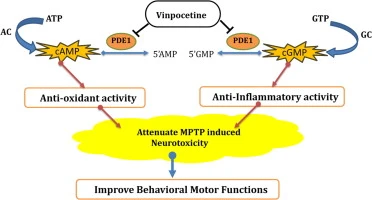
Vinpocetine Powder can improve cerebral microcirculation, inhibit neuroinflammation and enhance neuroprotection at both cellular and tissue levels through multi-target collaboration. The core mechanisms include blocking the voltage gate channel, inhibiting PDE1, and directly inhibiting IKK to down-regulate NF-κB-mediated inflammatory response. In addition, its main metabolite, cis‑apovincaminic acid, also showed neuroprotective activity in some models. The above-mentioned effects together explain its wide application in the research of cerebrovascular and neuroprotection.
Key molecular targets and modes of action
Voltage-gated Na+ channel blockade: Vinpocetine can inhibit abnormal depolarization and excitotoxicity of neurons and glial cells, and reduce the risk of Ca2+ influx overload, which is an important basis for its neuroprotection.
Synergism of metabolites: cAVA, its main metabolite, showed neuroprotective effect in NMDA-induced neurotoxicity model, suggesting that "parent drug+metabolite" may participate in the overall effect together.
The above points together outline the multi-target network of Vinpocetine, and extend the duration and breadth of protection through metabolites.
The chain of action from molecules to cells
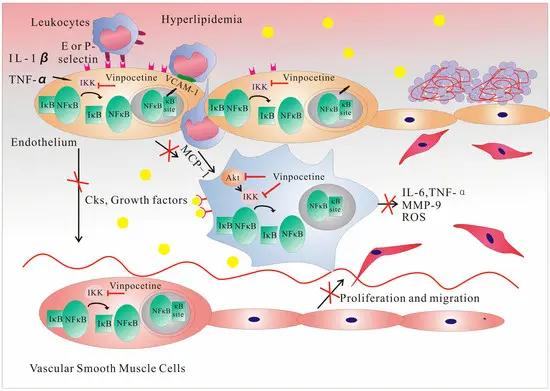
In vascular unit: promote vascular smooth muscle relaxation and improve endothelial function, thus enhancing cerebral microcirculation perfusion; This pathway is similar to the vasodilation logic of antihypertensive/antianginal drugs, but it emphasizes the cerebral circulation scene in this kind of research.
In the inflammatory unit: through IKK inhibition, the upstream signal of NF κ B was cut off, and various chemokines and adhesion molecules were significantly down-regulated, which reduced the adhesion and infiltration of leukocytes and alleviated the inflammatory damage of tissues; Vinpocetine Powder can reduce the infiltration of polymorphonuclear leukocytes in the induced lung inflammation model in mice.
In nerve cells: channel blocking reduces abnormal discharge and excitotoxicity, and cooperates with mediated neuroinflammation inhibition to reduce the risk of neuronal damage under ischemia/reperfusion or toxic stimulation; Vinpocetine and cAVA have protective effects on NMDA neurotoxicity in the rat model of olfactory cortex injury.
On the whole, it forms a closed-loop intervention in the three links of "blood vessel-inflammation-nerve", which not only improves blood supply, but also controls inflammation, and then reduces the excitotoxicity of neurons, thus enhancing the survival and functional preservation probability at the tissue level.
Tips on the use of experiments and preparations
Photosensitivity and stability: the product is sensitive to light, so the whole process should be operated and stored away from light as far as possible to reduce degradation and titer fluctuation caused by light.
The above-mentioned physicochemical and operational points are helpful to stably reproduce the biological effects of Vinpocetine in experiments and preparation development.
Action boundary and research orientation
Use boundary: There may be differences in impurity spectrum/crystal form/solvent residue between different batches and sources, so it is suggested to evaluate the consistency based on COA, methodology verification and stability data.
Applicable scenario: It is more suitable for studying the mechanism of cerebral microcirculation regulation, neuroinflammatory pathway, ion channel and PDE, as well as the pharmacodynamic evaluation and mechanism analysis of ischemia/reperfusion and neurotoxicity model.
Generally speaking, the value of Vinpocetine Powder is to provide a verifiable multi-target tool for the chain of "cerebrovascular-neuroinflammation-neuroprotection", but its clinical extrapolation still needs the support of higher quality human evidence.
From laboratory to factory: key points of technology and quality control of Vinpocetine Powder
Overview of process route
Synthesis route: Vinpocetine; was prepared from Vincamine by demethylation-esterification. The route is mature, the steps are simple, and it is suitable for large-scale amplification.
Key control: the quality of starting materials, reaction selectivity, solvent and catalyst residue, crystallization/recrystallization, drying and protection from light, metal and moisture, etc., determine the purity, crystal form and stability of the finished product.
Quality attribute and control strategy
Key quality attributes: Assay, related substances/degradation products, residual solvents, elemental impurities, moisture, melting point/optical rotation, crystal form/particle size, microbial limit/endotoxin (if applicable).
Key process parameters: temperature/time/material ratio, pH, crystallization procedure, drying curve, protection from light and inert gas, etc.
Analysis method of Vinpocetine Powder: HPLC was used to determine the content and related substances; Determination of residual solvent by GC; DSC/XRPD is used to confirm the crystal form and melting point; If necessary, establish particle size distribution and dissolution method.
Stability: carry out accelerated/long-term/intermediate condition stability, focusing on monitoring the growth, content change, appearance and dissolution of related substances; Establish re-inspection period/validity period and storage conditions (generally, powder is recommended to be-20 C, protected from light, dry and sealed).
Future trend: the evolution direction of technology, market and application
Technical trend
Green synthesis and continuity: reduce solvent consumption and "three wastes" emission, and improve energy efficiency and consistency.
Crystal form/particle size engineering: optimize dissolution and bioavailability by means of programmed cooling, seed method and airflow crushing.
Impurity spectrum: High sensitivity technologies such as HPLC-MS/GC-MS are used to establish the whole process impurity control and online monitoring.
market trend
Population aging and chronic disease management promote the growth of demand for cardiovascular and cerebrovascular related preparations; Investment in scientific research and demand for tool compounds remained stable.
Regionalized supply chain and localized production have become the trend, and enterprises need to find the optimal solution among quality, cost and delivery.
Differentiated products (such as specific crystal form/particle size and specific impurity spectrum) will become the focus of competition in the high-end market.
Application trend
In the fields of cerebrovascular disease, neuroinflammation and cognitive function, the research of Vinpocetine Powder will continue to deepen. At the preparation end, more attention will be paid to the correlation between release curve and clinic.
conclusion:
Although Vinpocetine is small, it is an important "cornerstone" in the field of brain health. From molecular mechanism to process quality control, from scientific research and application to pharmaceutical preparations, every step is related to safety, effectiveness and consistency. I hope this paper can help colleagues in R&D, procurement, quality and production to quickly establish a systematic understanding of Vinpocetine Powder with popular language and structured information, and make fewer detours and correct choices in practical work.
Xi'an Faithful BioTech Co., Ltd. combines cutting-edge production technology with comprehensive quality assurance to provide high-quality Vinpocetine Powder that meets international pharmaceutical standards. Our commitment to excellent, competitive prices and technical support makes us the preferred partner of global healthcare providers and researchers. Please contact our technical team in sales11@faithfulbio.com to find out how our products can improve your formula.
This is a list of the names of the core scientific research documents that I referred to and relied on in the process of writing a soft article. These documents provide solid scientific evidence for the efficacy and mechanism mentioned in this paper.
- Hagiwara M, et al. Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle. Biochemical Pharmacology, 33(3): 453–457, 1984.
- Zhang L, et al. Anti‑Inflammatory Effects of Vinpocetine in Atherosclerosis and Ischemic Stroke: A Review of the Literature. Molecules, 20(1): 335–347, 2014.
- Nyakas C, et al. Neuroprotective effects of vinpocetine and its major metabolite cis‑apovincaminic acid on NMDA‑induced neurotoxicity in a rat entorhinal cortex lesion model. CNS Neuroscience & Therapeutics, 15(2): 89–99, 2009.
- Souness JE, Brazdil R, Diocee BK, Jordan R. Role of selective cyclic GMP phosphodiesterase inhibition in the myorelaxant actions of M&B 22,948, MY‑5445, vinpocetine and 1‑methyl‑3‑isobutyl‑8‑(methylamino)xanthine. British Journal of Pharmacology, 98(3): 725–734, 1989.
- Kis B, et al. Vinpocetine is a neuroprotective drug that exerts beneficial effects on neurological symptoms and cerebrovascular disease. Journal of Neurochemistry, 124(2): 233–240, 2013.
- Piracetam and vinpocetine ameliorate rotenone‑induced Parkinsonism in rats. Indian Journal of Pharmacology, 44(6): 774–779, 2012.
- TSPO‑specific ligand vinpocetine exerts a neuroprotective effect by suppressing microglial inflammation. Neuron Glia Biology, 7(2–4): 187–197, 2011.
- [11C]Vinpocetine PET study on microglial activation evolution after stroke. Journal of the Neurological Sciences, 320(1–2): 110–117, 2012.
- Comparison of self‑microemulsifying drug delivery system versus solid dispersion technology used in the improvement of dissolution rate and bioavailability of vinpocetine. Acta Pharmaceutica Sinica, 2009, 44(6): 743–751.
- Surface‑modified emulsomes for intranasal delivery of drugs. US Patent US11234933B1, 2022‑02‑01.
- Vinpocetine product page – ATCC‑listed vendor (Shanghai Xinyu Biotech). Updated 2025‑05‑30.



