Why Dronedarone Hydrochloride Powder is a "potential stock" for treating atrial fibrillation?
What is Dronedarone Hydrochloride Powder?
In the "big family" of cardiovascular diseases, atrial fibrillation is definitely a headache "frequent visitor". To put it simply, atrial fibrillation is that the atrium above the heart beats too disorderly, like drumming, which not only makes people feel flustered, chest tightness and fatigue, but also may cause more dangerous thrombosis, stroke and even heart failure. One in every four adults in the world may experience atrial fibrillation in his life, and the elderly are the hardest hit.
In this context, as a new type of antiarrhythmic raw material powder, Dronedarone Hydrochloride Powder has gradually entered the public's field of vision. It is an "upgraded version" improved from "old star" Amiodarone, which focuses on "good curative effect and less side effects" What's so special about this small bag of white or white-like powder? Why is it so "exquisite" to use?
Dronedarone Hydrochloride is an antiarrhythmic product, which mainly acts on atrial Acetylcholine-dependent potassium channels, and has multi-channel inhibition. The simultaneous inhibition of inward and outward currents can reduce the transmural dispersion of repolarization, so the risk of arrhythmia is lower and it has better safety. Structurally, it is an optimization of the structure of Amiodarone, which belongs to the structural analogue of Amiodarone and aims to overcome iodine-related adverse reactions.
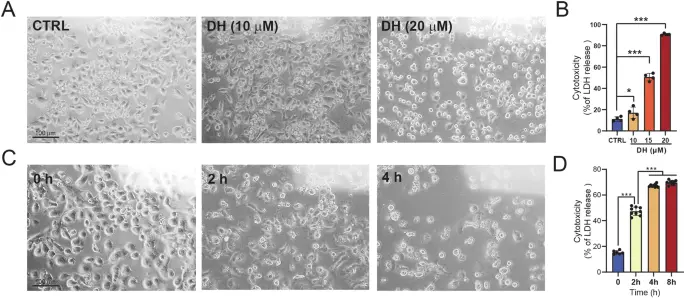
Where did it come from? -born to solve the pain point of "old medicine"
To understand it, we must start with its "predecessor" Amiodarone.
Amiodarone is the "main force" to deal with atrial fibrillation in the past few decades, especially for those whose heartbeat is dangerous and may even die suddenly. But it has a fatal flaw: it is too toxic! Because its molecules contain "iodine" atoms, long-term use will "accumulate" in the thyroid gland, lungs, liver, eyes and even skin, causing all kinds of troubles, and these side effects are often "slow fever", which may have caused irreversible harm to the body when discovered. What's more, Amiodarone "lingers" in the body-the half-life is as long as several tens of days.
Scientists have been thinking: "Can you transform Amiodarone, keep its effect of treating atrial fibrillation, but get rid of these terrible toxicity?"
As a result, pharmaceutical scientists began to "do it": they carefully studied the molecular structure of Amiodarone and found that "iodine atom" is probably the "culprit" of toxicity (especially thyroid and lung problems). If we can remove it and adjust other parts at the same time, can we reduce the toxicity?
After years of research, Dronedarone Hydrochloride Powder was born-it looks a bit like Amiodarone, but the key difference is that all iodine atoms were removed and other chemical structures were optimized (such as introducing methanesulfonyl groups and reducing fat solubility).
What changes have these changes brought about?
Toxicity is greatly reduced: Without iodine atom, the toxicity risk of thyroid and lung is obviously reduced (for example, hyperthyroidism/hypothyroidism is no longer easy to occur, and the probability of pulmonary fibrosis is lower).
Lower fat solubility: Amiodarone especially likes to drill into adipose tissue to accumulate, while Dronedarone Hydrochloride is more refreshing, and it is not easy to "hide dirt and accept dirt" in the body. Theoretically, tissue accumulation is less and half-life is shorter.
Multi-channel block: it still retains its original core ability-by blocking a variety of "current channels" in heart cells, it can restore the regularity of the jumping atrium.
Simply put, Dronedarone Hydrochloride is like an "upgraded antiarrhythmic product" that has been "refined": the goal is to fight atrial fibrillation while minimizing "accidental injury" to the body.
How is it produced?
You may think, "Isn't it just a bag of powder? What's so difficult? " But in fact, its production is a typical "high threshold, high technology" fine chemical and pharmaceutical process, and every step has to be "haggled over".
1. Synthesis: "Spell" molecules like Lego.
Its production began with a series of complex organic chemical reactions. Scientists need to start from basic chemical raw materials (such as simple organic compounds) and "build" the molecular skeleton of nedarone step by step through multi-step reactions (such as condensation, substitution and cyclization). This process is like Lego-every part (intermediate) must be accurately matched, and a slight deviation in reaction conditions (temperature, pressure, catalyst, pH value, etc.) may result in "defective products" (impurities).
2. Purification: Eliminate "impurities"
In the synthesized mixture, there will be many "by-products" (impurities) besides the target molecule (Dronedarone). However, these impurities may affect the effect and even bring additional risks. Therefore, we must use high-tech means (such as chromatographic separation and crystallization purification) to "find out" them and throw them away, leaving only Dronedarone Hydrochloride with high purity.

3. Salt formation and quality inspection: turning into a stable "powder"
The last step is to react the purified Dronedarone with organic matter to produce Dronedarone Hydrochloride. But it's not over yet-as an API, it must pass strict quality inspection, such as:
Identification: using infrared spectrum, mass spectrometry and other technologies to confirm that "this is Dronedarone Hydrochloride Powder, not a knockoff".
Content: Ensure the purity of the main components is between 98.0% and 102.0% (neither more nor less).
Related substances: detect and control the content of all impurities (for example, unknown impurities cannot exceed 0.1% and known impurities cannot exceed the standard).
Residual solvent: The organic solvent used in synthesis must be cleaned, and the residual amount must meet international standards.
Crystal form: the "crystal shape" of the product will affect the dissolution rate and absorption effect, and it must be guaranteed to be a stable and effective crystal form.
Only through all these "checkpoints" can this package of white or white-like powder be called "qualified Dronedarone Hydrochloride Powder".
What is its pharmacological action?
The pharmacological mechanism of Dronedarone Hydrochloride Powder is inherited from Amiodarone, which belongs to the third class of antiarrhythmic products, but it has the electrophysiological characteristics of all four kinds of antiarrhythmic products at the same time, and is a multi-channel blocker.
The main mechanism: by blocking the rapid delayed rectifier potassium current of myocardial cell membrane, the action potential duration and effective refractory period are prolonged, especially for atrial muscle. This is helpful to eliminate reentry excitation and prevent the recurrence of atrial fibrillation.
Auxiliary mechanism: At the same time, it also has the functions of slightly blocking sodium channels, uncompetitively antagonizing α and β adrenergic receptors and blocking L-type calcium channels. This multi-target effect enables it to effectively regulate the electrophysiological activities of atrium, atrioventricular node and ventricle, and achieve broad-spectrum antiarrhythmic effect.
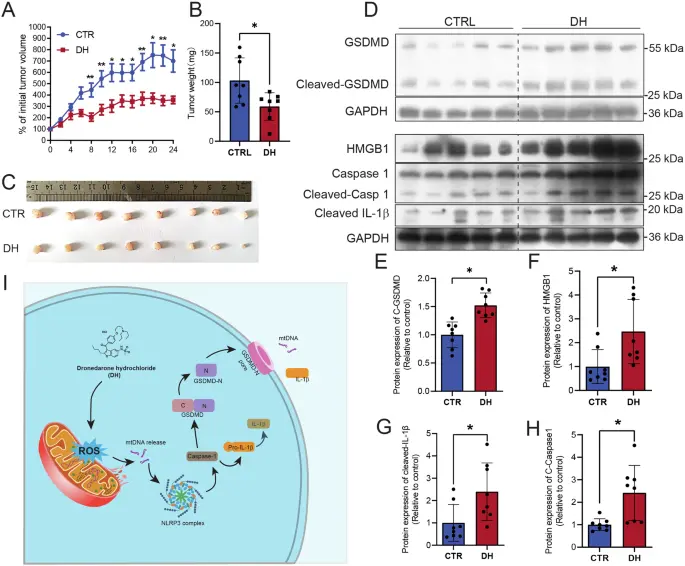
conclusion
It is undoubtedly a classic epitome of modern product research and development: it embodies the rational design idea of innovation based on traditional structure; It shows the superb skills of chemical synthesis and pharmaceutical technology; It warns us more deeply that the safety evaluation of a new product is a dynamic and continuous process from clinical trial to the wide application in the real world. Therefore, for the pharmaceutical industry, the story of Dronedarone Hydrochloride Powder the importance of targeted research and development and precise positioning. This small white powder bears not only the weight of chemical molecules, but also the awe of life and health, the exploration of scientific boundaries and the test of clinical wisdom.
Xi'an Faithful BioTech Co., Ltd. combines cutting-edge production technology with comprehensive quality assurance to provide high-quality Dronedarone Hydrochloride Powder that meets international pharmaceutical standards. Our commitment to excellent, competitive prices and technical support makes us the preferred partner of global healthcare providers and researchers. Please contact our technical team in sales11@faithfulbio.com to find out how our products can improve your formula.
This is a list of the names of the core scientific research documents that I referred to and relied on in the process of writing a soft article. These documents provide solid scientific evidence for the efficacy and mechanism mentioned in this paper.
- Sanofi. Multaq® (dronedarone) tablets Prescribing Information. U.S. Food and Drug Administration.
- European Medicines Agency. Assessment Report for Multaq (international non-proprietary name: dronedarone)
- Hohnloser, S. H., et al. (2009). Effect of dronedarone on cardiovascular events in atrial fibrillation. New England Journal of Medicine, 360(7), 668-678.
- Connolly, S. J., et al. (2011). Dronedarone in high-risk permanent atrial fibrillation. New England Journal of Medicine, 365(24), 2268-2276.
- Køber, L., et al. (2008). Increased mortality after dronedarone therapy for severe heart failure. New England Journal of Medicine, 358(25), 2678-2687.
- 《Martindale: The Complete Drug Reference》
- Dronedarone: Pharmacology, administration, and contraindications
- Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C., ... & ESC Scientific Document Group. (2021). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal, 42(5), 373-498.
- January, C. T., Wann, L. S., Calkins, H., Chen, L. Y., Cigarroa, J. E., Cleveland, J. C., ... & Yancy, C. W. (2019). 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Journal of the American College of Cardiology, 74(1), 104-132.
- The United States Pharmacopeial Convention. United States Pharmacopeia (USP) - Dronedarone Hydrochloride Monograph.
- Singh, B. N., Connolly, S. J., Crijns, H. J., Roy, D., Kowey, P. R., Capucci, A., ... & Hohnloser, S. H. (2007). Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. New England Journal of Medicine, 357(10), 987-999. (EURIDIS and ADONIS trials)
- Kowey, P. R., Dorian, P., Mitchell, L. B., Pratt, C. M., & Roy, D. (2009). Rationale and design of ATHENA: A placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Journal of Cardiovascular Pharmacology and Therapeutics, 14(3), 176-181.



