Bimatoprost API powder can really make eyelashes "grow against the sky"
Unexpected Beauty Discovery —— From Glaucoma to Eyelash Growth Liquid
1.1 A "beautiful mistake"
The story begins in the 1990s. At that time, ophthalmologists were looking for a more effective treatment for glaucoma. Glaucoma is called "the invisible thief of vision", which will gradually damage the optic nerve and lead to vision loss. Scientists found that prostaglandin analogues can effectively reduce intraocular pressure, so Bimatoprost API powder came into being.
In clinical trials, researchers noticed an interesting phenomenon: patients who used bimatoprost eye drops not only got their intraocular pressure under control, but their eyelashes became longer, thicker and darker! This unexpected "side effect" quickly attracted attention. Who would have thought that a product for treating eye diseases would become a revolutionary discovery in the beauty industry?
1.2 Deciphering the mechanism of action: the "awaker" of hair follicles
How does it "wake up" eyelash hair follicles? The scientific explanation is as follows:
Prolonging hair growth period: hair has a fixed growth cycle, including growth period, regression period and rest period. It can extend the growth period of eyelashes from about 30 days to about 45 days, so that eyelashes can grow for a longer time.
Stimulate the proliferation of hair follicles: it stimulates the proliferation of hair follicle cells by activating prostaglandin receptors, thus increasing the number of active hair follicles.
Increase pigmentation: it can also promote melanocyte activity, making new eyelashes darker and thicker.
Interestingly, these effects are reversible-once you stop using them, your eyelashes will gradually return to their original state, which explains why consumers will continue to buy related products.
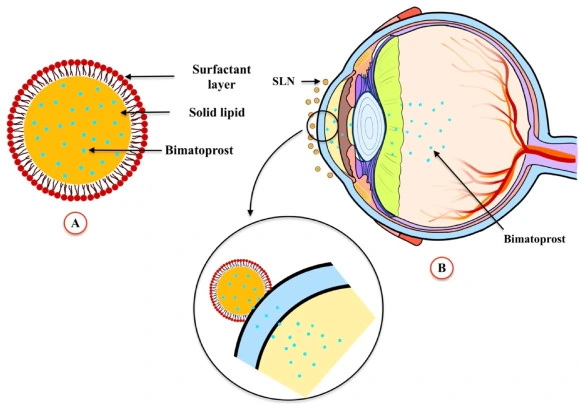
Bimatoprost API powder-the "core engine" of beauty revolution
2.1 what is API powder?
API is the abbreviation of "Active Pharmaceutical Ingredient". Bimatoprost API powder is a high-purity bimatoprost bulk drug, which is white or white-like crystalline powder. It is the "core engine" of all products containing Bimatoprost, and the beauty product Latisse (eyelash growth liquid) is inseparable from this magical powder.
2.2 Why is it in powder form?
You may be curious, why not directly produce finished products, but make raw material medicine powder? The reason is:
Stability: API in powder form is more stable than solution, which is convenient for long-term storage and transportation
Flexibility: different products need different concentrations of Bimatoprost, and the powder form can be accurately prepared.
Quality control: the quality inspection in the raw material stage is stricter to ensure the safety and effectiveness of the final product.
2.3 From raw materials to finished products: Bimatoprost's gorgeous transformation
Behind a small bottle of eyelash growth liquid is a complicated production process:
Synthesis of raw materials: Bimatoprost is synthesized by multi-step chemical reaction, which requires high-precision control of temperature, pressure and catalytic conditions.
Purification and crystallization: purification by recrystallization and other methods to remove impurities to obtain high-purity API powder. This step is very important, because even a small amount of impurities may cause adverse reactions.
Quality inspection: Advanced technologies such as high performance liquid chromatography (HPLC) and mass spectrometry are adopted to ensure that the purity is usually over 99.5%.
Formulation development: dissolve API powder in appropriate solvent, and add auxiliary ingredients such as stabilizer and preservative.
Aseptic filling: filling into special containers under strict aseptic environment.
In this process, the quality of API powder directly determines the safety and effectiveness of the final product, which is why global regulators have such strict requirements for API production.
Global market map
3.1 Distribution of Major Producing Countries
The production of Bimatoprost API powder is highly concentrated in several countries and regions:
Indian, China, Europe and America.
China: In recent years, it has developed rapidly in the field of API production. Many enterprises have obtained international certification, and the product quality has been significantly improved. Maintain technical advantages in the field of high-end API.
Europe and America: Although the overall share is small, it still maintains technical advantages in the field of patent API.
3.2 Consumer market trends
According to market research data, the global Bimatoprost market is expected to reach $1.87 billion by 2027, with a compound annual growth rate of about 6.2%. Among them:
Medical use: Glaucoma treatment is still the main application, especially as the global aging intensifies, the demand continues to grow.
Beauty uses: eyelash growth products have a faster market growth and have become one of the fastest growing segments of the beauty industry.
Technological Innovation and Future Trends
4.1 A new generation of prostaglandin analogues
Scientists are developing prostaglandin analogues with less side effects and better effects:
Selective receptor agonists: target specific receptors more accurately and reduce unnecessary side effects.
Local retention technology: improve the local retention time around the eyes and reduce systemic absorption.
Sustained-release preparation: develop a new preparation with a single use effect lasting for several weeks.
4.2 How to choose safe and effective Bimatoprost API powder products?
Check the certification: make sure the products come from legal and qualified manufacturers.
Check ingredients: avoid products with unknown ingredients or unclear concentration labels.
Professional consultation: especially those with eye diseases or sensitive skin, consult professionals and doctors before use.
Conclusion: the art of balance between beauty and science
The story of Bimatoprost API powder is a microcosm of the interweaving of modern science and human pursuit of beauty. From unexpected medical discovery to global beauty phenomenon, behind this small bottle of powder is complex chemical synthesis, strict quality control, the operation of global supply chain, and the continuous dialogue between science and ethics.
It reminds us that true beauty is never a simple "smear", but a balanced art based on scientific cognition, safety awareness and moderation principle. In the pursuit of long eyelashes, we should not ignore the fundamentals of eye health; While enjoying the beautiful possibilities brought by modern science and technology, we should keep rational judgment and careful choice.
Xi'an Faithful BioTech Co., Ltd. combines cutting-edge production technology with comprehensive quality assurance to provide high-quality Bimatoprost API powder that meets international pharmaceutical standards. Our commitment to excellent, competitive prices and technical support makes us the preferred partner of global healthcare providers and researchers. Please contact our technical team in sales11@faithfulbio.com to find out how our products can improve your formula.
This is a list of the names of the core scientific research documents that I referred to and relied on in the process of writing a soft article. These documents provide solid scientific evidence for the efficacy and mechanism mentioned in this paper.
- Woodward, D. F., et al. (2001). The pharmacology of bimatoprost (Lumigan™). Survey of Ophthalmology, 45(Suppl 4), S337-S345
- Cohen, J. L. (2010). Enhancing the growth of natural eyelashes: The mechanism of bimatoprost-induced eyelash growth. Dermatologic Surgery, 36(9), 1361-1371.
- United States Pharmacopeia - National Formulary (USP-NF).
- ICH Q7: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients
- Brandt, J. D., et al. (2001). Six-month comparison of bimatoprost once-daily and twice-daily with timolol twice-daily in patients with elevated intraocular pressure. Survey of Ophthalmology, 45(Suppl 4), S361-S368.
- Smith, S., et al. (2012). Eyelash growth in subjects treated with bimatoprost: A multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. Journal of the American Academy of Dermatology, 66(5), 801-806.
- Global Bimatoprost Market - Growth, Trends, COVID-19 Impact, and Forecasts (2023 - 2028)
- Active Pharmaceutical Ingredients (API) Market Analysis



