Cetrorelix acetate peptide powder: How to become a 'precise regulator' in assisted reproductive technology?
Cetrorelix acetate peptide is an artificially synthesized GnRH antagonist primarily used for controlled ovarian hyperstimulation (COH) therapy in assisted reproductive technology (ART). Its core function is to competitively inhibit the release of endogenous luteinizing hormone (LH), precisely regulate the follicular development cycle, and avoid treatment failure caused by premature ovulation. Compared to traditional drugs, Cetrorelix acetate peptide powder has the advantages of rapid onset, short treatment duration, and controllable side effects, especially suitable for patients with polycystic ovary syndrome (PCOS) or those who are intolerant to GnRH agonists. Clinical data shows that it can increase the success rate of multi follicle development by about 30%, becoming an indispensable "regulatory tool" in modern reproductive medicine.
Historical Development of Cetrorelix acetate peptide powder
The basis for developing GnRH antagonists was laid in 1971 with the structural elucidation and synthesis of GnRH by Schally and Guillemin . The GnRH is produced in a pulsatile manner by hypothalamic neurons in the area of the arcuate nucleus and controls pituitary-ovarian function. The main problem with gonadotropin stimulation is premature LH surge because of the positive feedback signal from estradiol to the pituitary gland. Consequences are premature ovulation, a reduction in oocyte and embryo quality, and, thus, a reduced pregnancy rate. Therefore, there was a need for further pharmacological research to develop a drug inhibiting a premature rise in LH . In 1981, the research continued with the synthesis of GnRH agonists, and in 1988, cetrorelix, the first GnRH antagonist, was discovered by Schally . In 1999, cetrorelix was the first “third generation" GnRH antagonist available on the European Union market for controlled ovarian stimulation in IVF and ICSI. One year later, ganirelix was approved in the EU as the second “third generation" GnRH antagonist, which was approved by the Food and Drug Administration in 1999. Important milestones for the clinical introduction of cetrorelix were the scientific works of Reissmann et al. who published study results about the clinical efficacy and effectiveness of cetrorelix. Diedrich et al. who published a dose-finding study for cetrorelix, provided further data for the clinical implementation of cetrorelix. Furthermore, Albano et al. published a prospective, randomized open-label study, establishing a multidose administration protocol for cetrorelix and demonstrating the noninferiority of cetrorelix in preventing an endogenous LH surge compared with the GnRH agonist buserelin. In the following years, cetrorelix was quickly established as a useful tool in reproductive medicine.
Comparison of the GnRH antagonist cetrorelix with GnRH analogs
cetrorelix was originally used to treat prostate carcinoma and benign prostatic hyperplasia. Cetrorelix is now 1 of the 2 injectable GnRH antagonists for controlled ovarian stimulation with assisted reproduction techniques. With this relatively young drug, the imminent complication of gonadotrophin stimulation ovarian hyperstimulation syndrome (OHSS) was reduced to almost zero in the short antagonist protocol because OHSS is triggered by human chorionic gonadotropin (HCG). In the short antagonist protocol, there is the possibility of triggering ovulation with a GnRH agonist, thus preventing HCG application . For this approach, 2 injections of the GnRH agonist triptorelin are performed usually about 36 hours before oocyte retrieval . Griesinger et al. in a new meta-analysis published in Human Reproduction Update in the same year, could show a comparable life birth rate between the 2 stimulation protocols. The European Society of Human Reproduction and Embryology recommended the short antagonist protocol as the standard stimulation protocol. This is primarily because of the greater safety of the patient. In addition, the stimulation duration and gonadotrophin consumption are reduced in the antagonist protocol. Whereas the number of oocytes retrieved is slightly reduced, there is equi-effectiveness in terms of oocyte quality and pregnancy rate Table 1 compares GnRH antagonists and GnRH agonists.
Action and clinical pharmacology
Mechanism of action: Targeted blockade of LH peak to ensure synchronous development of follicles
In the natural reproductive cycle, the hypothalamus produces GnRH which stimulates the pituitary to start secreting LH and FSH. The resulting LH surge then triggers ovulation.
Cetrorelix acetate peptide powder binds to the membrane receptors in the pituitary gland that are normally used by GnRH. By occupying these receptors, cetrorelix blocks endogenous GnRH from binding and stimulating the pituitary gland. This action leads to an immediate and dose-dependent suppression of LH and FSH secretion from the pituitary. The reduction in LH prevents the premature LH surge that typically leads to the release of eggs too early. By delaying the LH surge, cetrorelix allows follicles in the ovaries to grow and mature properly until it's time for egg retrieval during an ART cycle.This allows multiple follicles to mature and enables a timed oocyte retrieval during COS. When oocytes are ready for collection, recombinant human chorionic gonadotropin can be given to trigger final follicular maturation and luteinisation after stimulation of follicular growth.
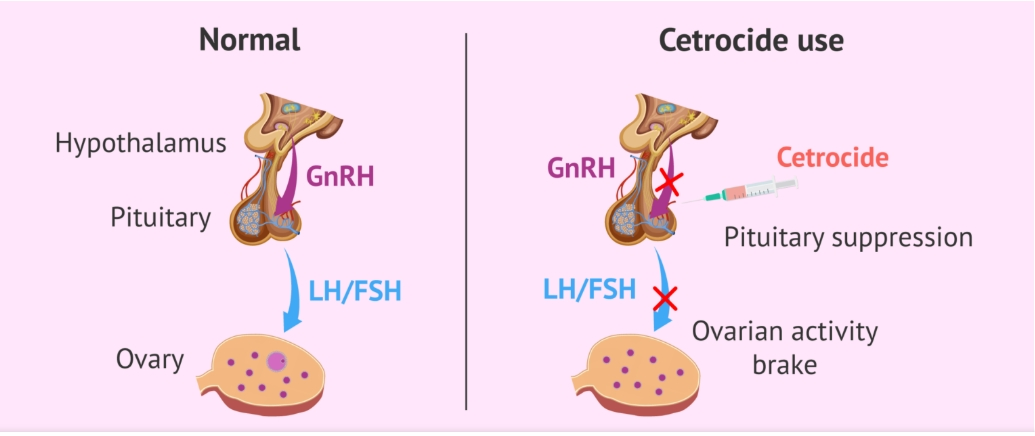
Unlike GnRH agonists that require a "desensitization" stage, Cetorelix acetate peptide does not need to wait for pituitary inhibition and can be used directly in the early stages of ovulation induction, shortening the treatment cycle. In addition, its half-life is moderate (about 6 hours), and stable blood drug concentration can be maintained through daily subcutaneous injection, providing doctors with a flexible medication plan.
Pharmacodynamics of Cetrorelix acetate peptide
GnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2) feedback at midcycle, GnRH liberation is enhanced resulting in an LH-surge. This LHsurge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and subsequently luteinization as indicated by rising progesterone levels.
cetrorelix competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a dose-dependent manner. The onset of LH suppression is approximately one hour with the 3 mg dose and two
hours with the 0.25 mg dose. This suppression is maintained by continuous treatment and there is a more pronounced effect on LH than on FSH. An initial release of endogenous gonadotropins has not been detected with cetrorelix which is consistent with an antagonist effect.
The effects of cetrorelix on LH and FSH are reversible after discontinuation of treatment. In women, cetrorelix delays the LHsurge, and consequently ovulation, in a dose-dependent fashion. FSH levels are not affected at
the doses used during controlled ovarian stimulation. Following a single 3 mg dose of cetrorelix duration of action of at least 4 days has been established.
A dose of cetrorelix 0.25 mg every 24 hours has been shown to maintain the effect.
Pharmacokinetics
The pharmacokinetic parameters of single and multiple doses of cetrorelix in adult healthy female subjects are summarized in the following Table
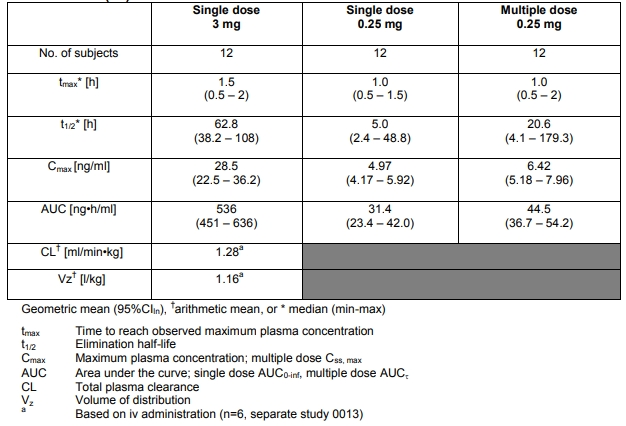
The pharmacokinetic parameters of single and multiple Absorption: cetrorelix is rapidly absorbed following subcutaneous injection, maximal plasma concentrations being achieved approximately one to two hours after administration. The mean absolute bioavailability of cetrorelix following subcutaneous administration to healthy female subjects is 85%.
Distribution: The volume of distribution of cetrorelix following a single intravenous dose of 3 mg is about 1 L/kg. In vitro protein binding to human plasma is 86%.
cetrorelix concentrations in follicular fluid and plasma were similar on the day of oocyte pick-up in patients undergoing controlled ovarian stimulation. Following subcutaneous administration ofcetrorelix 0.25 mg and 3 mg, plasma concentrations of cetrorelix were below or in the range of the lower limit of quantitation on the day of oocyte pick up and embryo transfer.
Metabolism: After subcutaneous administration of 10 mg cetrorelix to females and males, cetrorelix and small amounts of (1-9), (1-7),(1-6), and (1-4) peptides were found in bile samples over 24 hours.
Clinical Advantage: Dual Breakthrough in Safety and Efficacy
1. Reduce OHSS risksMechanism validation:
cetrorelix reduces the excessive response of follicular granulosa cells to hCG by inhibiting endogenous LH peaks. A multicenter randomized controlled trial (RCT) showed that the incidence of OHSS in the antagonist regimen group was 3.2%, significantly lower than the 6.5% in the traditional rectangular regimen group (P<0.01).High risk population protection: In high response patients with AMH ≥ 5.5 ng/mL, antagonist regimens reduced the risk of severe OHSS from 12% to 2.8% (OR=0.23, 95% CI 0.09-0.58).
2. Improve embryo quality
Follicle synchronous development: The cetrorelix regimen can increase the synchronicity of follicles ≥ 14mm by 40%, and the proportion of high-quality oocytes increases from 58% to 72% (P<0.05)
Pregnancy rate improvement: After combining blastocyst culture technology, the clinical pregnancy rate of the antagonist regimen (48.7%) was significantly higher than that of the traditional regimen (39.2%), and the difference was statistically significant (P=0.003).
3. Applicability and safety
High response patient efficacy: In PCOS patients, the pregnancy rate of the antagonist combined with low-dose FSH regimen (52.1%) is comparable to the standard protocol, but the risk of OHSS is reduced by 60%.
Drug interactions: In vitro metabolic studies have confirmed that Cetrotide has no competitive binding sites with FSH, and there is no significant change in blood drug concentration when used in combination (Cmax difference<5%).
Who and when to use Cetrorelix peptide powder?
Who can use?
Polycystic ovary syndrome (PCOS) patients: High LH levels can easily lead to uneven follicular development, and cetrorelix acetate can effectively correct endocrine disorders.
Repeated IVF failure: By optimizing the follicular development environment and improving embryo implantation conditions.
Elderly or those with low ovarian reserve function: shorten the ovulation induction cycle and reduce the burden of ovarian stimulation.
For GnRH agonist intolerant individuals: avoid side effects such as withdrawal bleeding caused by agonists.
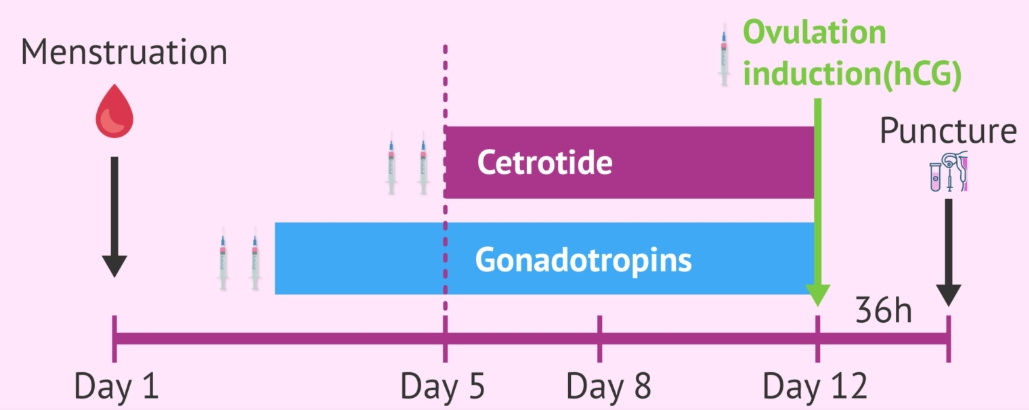
When is Cetrorelix peptide administered?
Cetrorelix acetate peptide powder is used towards the end of in vitro fertilization (IVF) cycle, from the fifth or sixth day of controlled ovarian stimulation until the day of the follicular puncture.In summary, a woman must first be given a drug containing gonadotropins (FSH and LH) to help her ovarian follicles mature.Then, once the follicles have reached a certain size, Cetrotide is applied so that they do not grow excessively and are kept in the ideal size (over 16-18 millimetres), as well as preventing the premature release of the eggs
Future prospects: Potential roles in personalized medicine
With the development of precision reproductive medicine, the application scenarios of cetuximab acetate will be further expanded:
Genetic testing guidance for medication: By analyzing the patient's genetic polymorphism, optimize the dosage and course of treatment of antagonists.
Innovative combination therapy: Combining with anti Mullerian hormone (AMH) detection to achieve dynamic prediction of ovarian response.
Assisted treatment for male infertility: exploring its potential value in regulating male sex hormones.
Conclusion
Cetrorelix acetate peptide powder, with its precise regulatory mechanism and clinically validated efficacy, has become an indispensable "golden partner" in assisted reproductive technology. Whether it is improving the success rate of treatment or ensuring patient safety, it always takes science as the cornerstone, lighting up the hope of childbirth for countless families.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality Cetrorelix acetate peptide raw materials that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of Cetrorelix acetate peptide powder, please contact our technical team through the following methods: sales12@faithfulbio.com
Reference
1.Cetrotide® (cetrorelix acetate) EU Product Information. January 2018.
2.Tur-Kaspa I and Ezcurra D. Expert Opin Drug Metab Toxicol 2009;5:1323–1336
3.Holesh J and Lord M. Physiology, Ovulation. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441996/#_NBK441996_pubdet_. Accessed: March
4.Guillemin R. Purification, isolation, and primary structure of the hypothalamic luteinizing hormone-releasing factor of ovine origin. A historical account. Am J Obstet Gynecol. 1977;129:214–218. doi: 10.1016/0002-9378(77)90749-9
5.Schally A.V. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315–322. doi: 10.1055/s-2008-1073142
6.Devroey P., Polyzos N.P., Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26:2593–2597. doi: 10.1093/humrep/der251.
7.Devroey P., Adriaensen P. OHSS Free Clinic. Facts Views Vis Obgyn. 2011;3:43–45.



