Have you found the ultimate ingredient for the next generation of scientific weight loss? Retatrutide Raw Powder is reshaping the health industry!
1.The development process of Retatrutide Raw Powder
1.1R&D Background
As the global obesity rate continues to rise, chronic diseases such as metabolic disorder, cardiovascular disease and type 2 diabetes caused by obesity expose hundreds of millions of people to health risks. The emergence of GLP-1 agonist drugs has brought breakthroughs in anti obesity treatment, but researchers are still seeking more potent next-generation drugs. Retatrutide Raw Powder was developed by Eli Lilly in the United States in this context.
1.2Key R&D Stage
Preclinical studies: Retarutide has been designed as a triple agonist targeting glucose dependent insulinotropic peptides, GLP-1, and glucagon receptors. It is based on a heavily modified GIP peptide backbone and is a fatty acid acylated single peptide that combines GCGR, GIPR, and GLP-1R activity. It can stay in the bloodstream for a long time and only needs to be taken once a week.
Phase II clinical trial: 338 adult obese patients were enrolled in the Phase II clinical trial to investigate the weight loss effects of three doses of 4mg, 8mg, and 12mg, as well as different starting doses. The results showed that at 24 weeks of treatment, Retatrutide (1mg, 4mg, 8mg or 12mg) reached the primary end point of efficacy evaluation in obese or overweight adult patients (except diabetes), with an average weight loss of 17.5%. At 48 weeks of treatment, participants who received weekly injections of 12mg Retarutide experienced an average weight loss of 24.2%. In addition, Eli Lilly has previously released phase II clinical data on non-alcoholic steatohepatitis (NASH). At treatment doses of 8mg and 12mg, the average relative liver fat loss rate of patients was greater than 80%, with over 80% of patients experiencing a fat loss rate of 70% or higher. At 48 weeks, liver steatosis disappeared in over 85% of patients.
Phase III clinical trials: There will be multiple Phase III clinical updates by 2025. On September 10th, Eli Lilly registered a phase III clinical trial of Retatrutide for the treatment of MASLD (metabolic associated fatty liver disease) on the Clinicaltrials.gov website. The phase III trial plans to enroll 4500 MASLD patients and is expected to be completed by 2030; On June 24th, Lilly also registered a phase III clinical trial of Retarutide for the treatment of obesity with lower back pain. The trial plans to enroll 586 obese patients with lower back pain, with a follow-up period of 80 weeks, and is expected to be completed by September 2027. At present, retarutide has a number of Phase III clinical trials in progress for obesity, type II diabetes and other indications .

1.3Research and Development Prospects
Retatrutide has shown remarkable weight loss and liver fat improvement effects in clinical trials. If subsequent clinical trials are successful and approved for market, it is expected to become a star drug in the field of anti obesity treatment, bringing more effective treatment options for obese patients. At the same time, it also provides a successful example for the development of multi-target drugs, promoting the development process of anti obesity and related metabolic disease treatment drugs in the pharmaceutical industry.
2.The effect of Retatrutide Raw Powder on weight loss
2.1Significant weight loss effect
Retatrutide has shown remarkable performance in weight loss, with good data from clinical trials at different stages. For example, in the Phase 2 clinical trial, after 48 weeks of treatment, patients in the 12mg dose group experienced an average weight loss of 24.2%. In the Phase 3 clinical trial, the results were even more astonishing. In the trial targeting obese and knee osteoarthritis patients, patients who received the highest dose of Retarutide treatment for 68 weeks lost an average weight of 28.7%, approximately 71.2 pounds. This is much more effective than Lilly's already listed Zepbound (about 22.5%) and competitor Novo Nordisk's Wegovy (less than 20%).
2.2Compared with other weight loss drugs, it has significant advantages
Compared with common weight loss drugs such as semaglutide and tilpotide, Retatrutide has obvious advantages. The weight loss effect of semaglutide is about 15% in one year, while that of tilpotide is about 20%, and the weight loss effect of Retatrutide in phase 3 clinical trials far exceeds them. This also makes Retatrutide highly competitive in the weight loss drug market, providing better options for obese patients.
2.3The impact on the weight loss drug market
The excellent weight loss effect of Retatrutide has caused strong reactions in the capital market. After the release of relevant experimental data by Eli Lilly, the company's stock price rose by 1.2% in pre-market trading and closed up 1.6% overnight. This demonstrates the high recognition and expectations of the market for Retatrutide, which is likely to open up a new heavyweight product line for Lilly and continue its growth momentum in the field of weight loss drugs.
Overall, Retatrutide Raw Powder has outstanding weight loss effects, and if it can be successfully approved for market in the future, it will definitely bring new hope to many obese patients.

3.The effect of Retatrutide Raw Powder on metabolism
The impact on weight: Multiple clinical trials have shown that Retarutide has a significant effect on weight loss. In a 48 week Phase 2 obesity study, taking 8mg and 12mg of Retatrutide resulted in a weight loss of 22.8% and 24.2%, respectively. According to Phase 3 clinical data released by Eli Lilly, patients with obesity and knee osteoarthritis who received the highest dose of Retarutide treatment for 68 weeks experienced an average weight loss of 28.7%. The principle is to activate glucagon receptors, promote the breakdown of stored fat (fat breakdown), and stimulate thermogenesis (thermogenesis), thereby increasing calorie burning; Simultaneously activating GLP-1 receptors in the brain can reduce appetite, increase satiety, and reduce food intake.
The impact on blood sugar: Retarutide can stimulate the pancreas to release insulin when blood sugar rises, while inhibiting the release of glucagon (a hormone that raises blood sugar), which helps to better control blood sugar. GIP works synergistically with GLP-1 to stimulate pancreatic insulin secretion in a glucose dependent manner and enhance the body's response to insulin, promoting better tissue absorption of glucose.
The impact on liver fat: Retatrutide has shown excellent performance in the treatment of metabolic dysfunction associated fatty liver disease (MASLD, formerly known as non-alcoholic fatty liver disease). At 24 weeks, the 8mg and 12mg dose groups showed a relative reduction of 80% in liver fat, and the therapeutic effect persisted at 48 weeks, with the highest reduction reaching 86%.
Positive impact: Retatrutide brings new hope for the treatment of metabolic diseases such as obesity, diabetes and non-alcoholic fatty liver disease. Its significant weight loss and improvement of metabolic indicators may provide patients with more effective treatment options and reduce the risk of related complications.
Potential risks: The use of Retarutide may also bring some side effects, the most common of which are gastrointestinal problems, including nausea, diarrhea, vomiting, and constipation, mostly mild to moderate. Some subjects discontinued treatment in phase 3 clinical trials due to excessive weight loss.
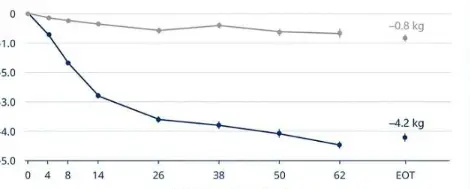
4.What is the mechanism by which Retarutide Raw Powder affects appetite
4.1Activate GLP-1 receptor
Retatrutide can activate GLP-1 receptors in the brain, thereby reducing appetite and increasing satiety. When it binds to GLP-1 receptors, it slows down gastric emptying. Imagine that the stomach is like a pocket, and as food stays inside for a longer period of time, it sends signals to the brain, telling it that it has already eaten a lot and no longer needs to eat, thereby reducing food intake and achieving the goal of controlling appetite.
4.2Acting on GIP receptors
GIP receptors exist in the central nervous system, as well as in the entire gastrointestinal tract and body, playing an important role in the central mechanisms that affect hunger, and can also send satiety signals from the gastrointestinal tract to the brain. After binding to GIP receptors, Retatrutide can enhance insulin secretion, synergize with GLP-1, further regulate blood sugar, and also help increase satiety and reduce appetite.
4.3Activate glucagon receptor
Glucagon receptors are mainly present in the liver and kidneys. After binding to this receptor, Retatrutide increases the production of glucagon, stimulates the breakdown of stored forms of energy such as fat and glycogen, and increases the body's basal metabolism. Even during sleep, the body is consuming more energy, which reduces its demand for food and suppresses appetite.
Conclusion
Overall, Retatrutide has shown great potential in regulating metabolism, but it is still in the clinical trial stage. If you are interested in this drug, it is recommended to closely monitor its research progress and learn about it under the guidance of a professional doctor. In the future, with the deepening of research, Retatrutide is expected to bring new breakthroughs in the treatment of metabolic diseases.
Xi'an Faithful BioTech provide you with the highest quality Retatrutide Raw Powder ,raw powder,Purity>99%.Please contact me!E-mail :sales10@faithfulbio.com .
Reference
1. Retatrutide for the treatment of metabolic dysfunction related fatty liver disease [June 24, 2024]
2. Retarutide/Retarutide/Retarutide/Retarutide [2014-11-17]
3. Retatrutide [June 13, 2025]
4. Retarutide: Losing 24.2% weight at 48 weeks may reverse fatty liver [June 27, 2023]
After one year of treatment, the average weight loss was 28.7%! The new generation of weight loss drugs from Eli Lilly has shown amazing performance and far superior efficacy ..[2025-12-12]
6. Three target agonists, retatrutide, achieve 100% weight loss goals and can also lower blood sugar and fat! Next [June 27, 2023]
7. Retarutide (LY3437943) TFA is a glucagon receptor (GCGR) and glucose dependent promoter
8. The new generation of weight loss drugs can significantly reduce weight, and the side effects are also significant [2020-02-21]
9. Retalutide, Retatrutide,2381089-83-2[2023-10-24]
10. The latest data on the three receptor agonist Retatrutide has been released [July 3, 2023]
11. The latest data on the three receptor agonist Retatrutide has been released! [2023-07-03]
12. The effect of endotoxins on metabolism [223-11-28]
13. Metabolic factors [February 15, 2024]
14. Retarutide. The most detailed user manual for Retarutide is available on August 12, 2025
15. New therapies for metabolic related fatty liver disease: empagliflozin Saroglitazaar、Retatrutide...[2023-11-13]
16. Multi omics mechanism study on the improvement of ethanol induced acute oxidative stress by regulating the gut fungal metabolite gene network through the extract of Gesneria diffusa [225-10-31]
17. Retarutide (LY3437943) | Retarutide
18. Retarutide is a three target receptor agonist similar to Somalutide [2024-11-10]
19. Retarutide (LY3437943) | GCGR/GIPR/GLP-1R agonist
20. Retatrutide[2024-07-26]
21. Losing 37 pounds in 6 months! Is Retatrutide ushering in the era of weight loss' triple receptor agonists'? NEJM has published the latest ..[2023-08-22]



