How can Entacapone API powder optimize the efficacy of levodopa in the treatment of Parkinson's disease?
Entacapone API powder is mainly used as an adjuvant therapy for Parkinson's disease (PD) patients. Since its first approval in 2000, Entacapone has been widely used in clinical practice and as part of compound formulations, significantly improving patients' quality of life. According to a retrospective study in the New England Journal of Medicine (NEJM) in 2023, Entacapone combined with Levodopa can reduce the daily "off period" time of PD patients by about 1.5 hours, and the safety data is consistent with earlier studies. In recent years, its indications have been extended to early PD patients to delay disease progression. In terms of global market, IQVIA data shows that the sales of Entacapone drugs reached 1.23 billion US dollars in 2022, with compound preparations accounting for over 60%. Today, let's take a look at how Entacapone optimizes the efficacy of Levodopa.
What is Entacapone?
Entacapone, is a medication commonly used in combination with other medications for the treatment of Parkinson's disease. Entacapone together with levodopa and carbidopa allows levodopa to have a longer effect in the brain and reduces Parkinson's disease signs and symptoms for a greater length of time than levodopa and carbidopa therapy alone.
Entacapone is a selective and reversible inhibitor of the enzyme catechol-O-methyltransferase (COMT). When taken together with levodopa (L-DOPA) and carbidopa, entacapone stops COMT from breaking down levodopa, resulting in an overall increase of levodopa remaining in the brain and body. Entacapone does not cross into the brain and hence does not inhibit COMT there。It is a kind of greenish yellow to yellow powder.
What is Entacapone used for?
Entacapone is used in addition to levodopa and carbidopa for people with Parkinson's disease to treat the signs and symptoms of end-of-dose "wearing-off.""Wearing-off" is characterized by the re-appearance of both motor and non-motor symptoms of Parkinson's disease occurring towards the end of a previous levodopa and carbidopa dose.
A double-blind, placebo controlled trial of PD patients with motor fluctuations on levodopa/carbidopa revealed that the addition of entacapone compared to placebo provided a significant increase in ‘on' time per day of approximately 1 h, while another study using a similar protocol demonstrated an increase in the mean daily ‘on' time by 1.4 h. Both studies allowed a 12% reduction in levodopa dose. Other studies in patients with motor fluctuations demonstrated that the addition of entacapone to levodopa increased ‘on' time and improved motor function as measured by the UPDRS and increased quality of life. In patients with motor fluctuations on levodopa, the addition of either entacapone or cabergoline (a long acting dopamine agonist) similarly improved UPDRS scores, quality of life as measured by the Parkinson Disease Questionnaire (PDQ)-39, and reduced daily ‘off' time. However, the reduction in ‘off' time occurred faster in the entacapone group than the cabergoline group. Entacapone has also been demonstrated to provide some benefit for patients who have a stable response to levodopa (i.e., not experiencing motor fluctuations), including motor improvement and improvement in several quality of life measures. Entacapone is indicated as an adjunct to levodopa to treat patients experiencing end-of-dose wearing-off. It has no antiparkinsonian effect on its own. It can be combined with immediate or sustained release levodopa/carbidopa.
How Entacapone optimize the efficacy of levodopa?
Entacapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT). COMT eliminates biologically active catechols present in catecholamines (dopamine, norepinephrine, and epinephrine) and their hydroxylated metabolites. When administered with a decarboxylase inhibitor, COMT acts as the major metabolizing enzyme for levodopa and metabolizes it to 3-methoxy-4-hydroxy-L-phenylalanine (3-OMD) in the brain and in the periphery.
Entacapone is peripherally selective and inhibits COMT in the body but not in the brain. As a result, entacapone inhibits the peripheral metabolism of levodopa, thus increasing plasma levels of levodopa. This causes more constant dopaminergic stimulation in order to reduce the signs and symptoms presented in the disease.
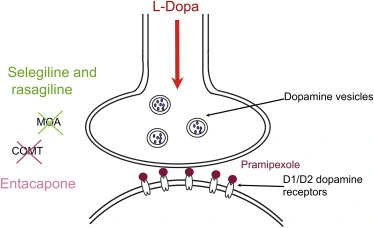
What is the defferences between Entacapone and tolcapone?
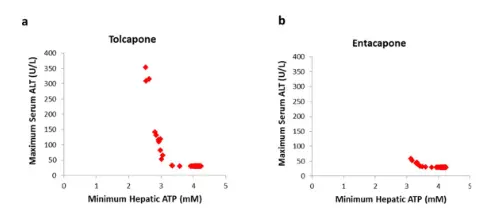
Entacapone and tolcapone are reversible COMT inhibitors which have been approved for clinical use in patients with Parkinson disease (PD). Nebicapone is a third COMT inhibitor which has been studied in humans. COMT inhibitors are used in combination with levodopa and a dopa decarboxylase (DDC) inhibitor. Each of them has problems either in pharmacokinetics, pharmacodynamics, clinical efficacy, or in safety. All three inhibitors have short elimination half-lives, about 2–3 h. Tolcapone is longer acting and more potent COMT inhibitor than entacapone; nebicapone lies in between. However, none of the present inhibitors cause a complete peripheral COMT inhibition. Tolcapone and nebicapone have increased more levodopa AUC than entacapone which is reflected also in their clinical efficacy. The most common adverse event with COMT inhibitors is dyskinesia which is usually managed by decreasing levodopa dose. The greatest problem with tolcapone and probably also with nebicapone is their liver toxicity which is not seen with entacapone. Tolcapone causes severe diarrhea more often than entacapone. Though the present COMT inhibitors have improved significantly the treatment of advanced PD patients, they still have several problems and weaknesses leaving room for developing better COMT inhibitors.
Entacapone and tolcapone are COMT inhibitors and prevent levodopa metabolism to 3-O-methyldopa, thus extending the half-life of levodopa. Entacapone acts only peripherally, whereas tolcapone has a central effect too. The former is used more widely, as the latter can be associated with liver toxicity.
Unlike tolcapone, Entacapone API powder does not cross the BBB. It is readily absorbed across the intestinal mucosa and is highly protein bound, with a plasma elimination half-life of 0.4–0.7 h. Entacapone 200 mg given 4–6 times daily with levodopa produced a 33% reduction in COMT activity. The administration of entacapone with levodopa/carbidopa immediate release (IR) increases levodopa AUC by 35–40%, and prolongs the levodopa half-life by 1.3–2.4 h. Entacapone does not significantly influence levodopa Tmax and Cmax. A similar pharmacokinetic effect is seen when entacapone is used with levodopa/carbidopa controlled release.
Will Entacapone react with other drugs?
In studies, entacapone has shown a low potential for interaction with other drugs. In theory, it could interact with MAO inhibitors, tricyclic antidepressants and noradrenaline reuptake inhibitors because they also increase catecholamine levels in the body, with drugs being metabolized by COMT with iron because it could form chelates, with substances binding to the same albumin site in the blood plasma (for example ibuprofen), and with drugs being metabolized by the liver enzyme CYP2C9 . None of the medications tested in studies have shown clinically relevant interactions, except perhaps warfarin for which a 13% (CI90: 6–19%) increase in INR was seen when combined with entacapone.
Are there any other potential applications for Entacapone?
Entacapone reduced muscle atrophy associated with hyperlipidemia
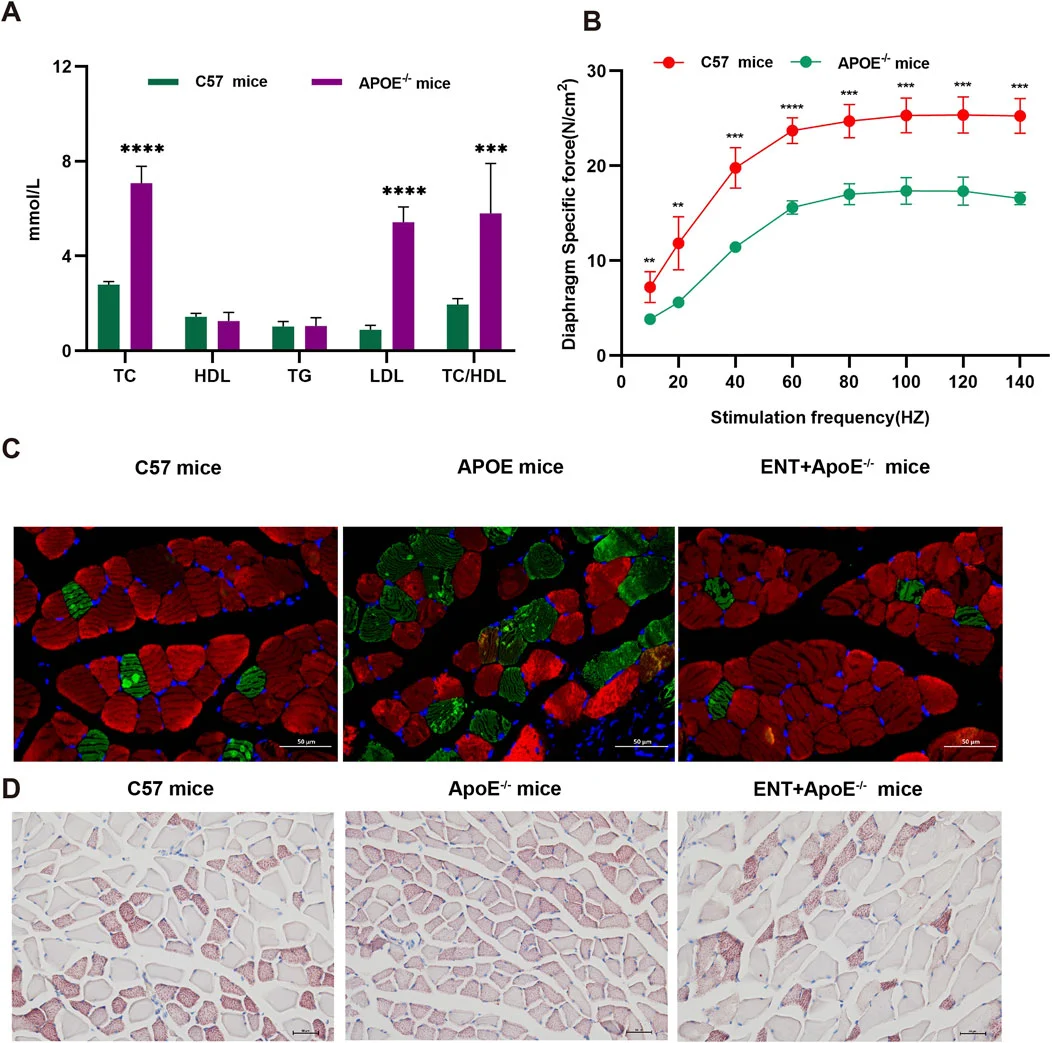
To evaluate the impact of hyperlipidemia on muscle atrophy and the potential effects of Entacapone on the muscles of mice with hyperlipidemia, A study employed APOE−/− mice and measured blood lipid levels. The results indicated that APOE−/− mice exhibited higher levels of TC, LDL, and TC/HDL compared to C57 mice. Furthermore, muscle strength was reduced in hyperlipidemic mice relative to C57 mice . Concurrently, immunofluorescence analysis of the gastrocnemius muscle revealed a smaller CSA of gastrocnemius myofibers in APOE−/− mice compared to C57 mice, suggesting myofiber atrophy. Notably, the administration of Entacapone mitigated this myofiber atrophy . Lipid content was assessed through Oil Red O staining, which corroborated the reduction in gastrocnemius muscle CSA in APOE−/− mice and demonstrated that ENT treatment protected against muscle fiber atrophy. Additionally, APOE−/− mice exhibited more lipid droplet accumulation in muscle compared to C57 mice, while treatment with Entacapone significantly alleviated lipid aggregation in the gastrocnemius muscle of APOE−/− mice . Taken together, findings demonstrate that Entacapone treatment confers a protective effect against muscle atrophy associated with hyperlipidemia.
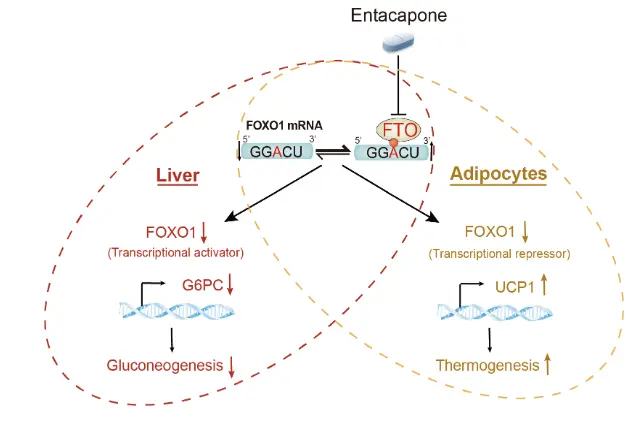
Entacapone may help with weight loss
A study led by Dr. HUANG Niu at National Institute of Biological Sciences and Dr. YANG Yungui at Beijing Institute of Genomics of Chinese Academy of Sciences has revealed how entacapone, a FDA proved drug for Parkinson's disease, regulates energy homeostasis and body weight through inhibiting FTO's demethylation activity on m6A. researchers discovered that entacapone can directly inhibit the demethylation activity of FTO. Researchers used adenovirus system to integrate G6PC promoter-luciferase reporter gene into the mouse liver to monitor the in vivo luciferase signal to find specific mechanism:FTO inhibition by entacapone upregulates m6A level in FOXO1 mRNA leading to the downregulated protein abundance of FOXO1.
What are the side effect of Entacapone?
The following side effects have been reported by people with Parkinson's disease treated with entacapone:Abdominal pain、Nausea、Vomiting、Fatigue、Dry mouth and Back ache.
Movement problems
The most common side effect of entacapone is movement problems, which occur in 25% of people taking entacapone. This drug may cause or worsen dyskinesia for people with Parkinson's disease treated together with levodopa and carbidopa.
Diarrhea
10% of patients taking entacapone have been shown to experience diarrhea. Diarrhea may occur within 4–12 weeks of initial entacapone use but resolves after discontinuation of the drug.
Urine color
10% of people taking entacapone experience a change in urine color to orange, red, brown, or black. This side effect is due to entacapone metabolism and excretion in the urine and shown to not be harmful.
Sudden sleep onset
People have reported sudden sleep onset while engaging in daily activities without prior warning of drowsiness. In controlled studies, patients on entacapone had a 2% increased risk of somnolence compared to placebo.
Low blood pressure
Episodes of orthostatic hypotension have been shown to be more common at the start of entacapone use due to increased levels of levodopa.
Conculsion
Entacapone API powder, as an important adjuvant drug for the treatment of Parkinson's disease, significantly improves patients' motor function by optimizing the pharmacokinetic properties of Levodopa. In terms of safety, although Entacapone has a low risk of liver toxicity (incidence<2%), it is necessary to strictly follow the medication guidelines of FDA and EMA and regularly monitor liver enzyme levels. In the future, with the advancement of clinical trials of new COMT inhibitors, there is still room for optimization of Entacapone powder, but its position as a classic drug is difficult to shake in the short term.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality Entacapone API powder, that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of Entacapone , please contact our technical team through the following methods:sales12@faithfulbio.com.
Reference
1 DM Longo 1,2 , Y Yang 1, PB Watkins 1,2 , BA Howell 1,2 and SQ Sile Elucidating Differences in the Hepatotoxic Potential ofTolcapone and Entacapone With DILIsymVR, a MechanisticModel of Drug-Induced Liver Injury CPT Pharmacometrics Syst. Pharmacol. (2016) 5, 31–39; doi:10.1002/psp4.12053.
2 Rong Zeng;Hanbing Xu;Mingzheng Wu;Xianlong Zho ect Entacapone alleviates muscle atrophy by modulating oxidative stress, proteolysis, and lipid aggregation in multiple mice models Front. Physiol., 09 December 2024.
3 Chinese Academy of Sciences Entacapone Identified as Chemical Inhibitor of Fat-mass and Obesity-associated Gene Mediating Metabolic Regulation 2019.4.17 oline.
4. "Comtan Full Prescribing Information-Novartis" (PDF). Pharma.us.novartis.com. July 2014. Archived from the original (PDF) on 15 March 2016. Retrieved 4 November 2015.
5. Koda-Kimble MA (2013). Koda-Kimble & Young's Applied Therapeutics: The Clinical Use of Drugs. Philadelphia: Lippincott Williams & Wilkins. pp. 1373–1374. ISBN 978-1609137137.
6. "Comtan: EPAR – Product Information" (PDF). European Medicines Agency. 10 March 2015. Archived from the original (PDF) on 16 March 2018. Retrieved 17 April 2017.



