How Does 6-Diazo-5-Oxo-L-Norleucine Affect Glutathione Production?
6-Diazo-5-Oxo-L-Norleucine represents a critical research compound that profoundly influences cellular glutathione biosynthesis through its unique mechanism as a glutamine analog. This potent inhibitor disrupts glutamine-dependent metabolic pathways, consequently affecting the production of glutathione, one of the most important antioxidant molecules in cellular systems. Understanding how 6-Diazo-5-Oxo-L-Norleucine interferes with glutathione synthesis requires examining its competitive inhibition of glutamine-utilizing enzymes, particularly glutaminase and gamma-glutamylcysteine synthetase. The compound's ability to modulate glutathione levels has far-reaching implications for cancer research, metabolic studies, and therapeutic development. By targeting the glutamine dependency of various cellular processes, this compound provides researchers with a powerful tool for investigating oxidative stress responses and cellular defense mechanisms in both normal and pathological conditions.
Molecular Mechanisms of Glutathione Synthesis Inhibition
Glutamine Analog Competition
6-Diazo-5-Oxo-L-Norleucine exerts its primary effect on glutathione production through competitive inhibition of glutamine-dependent enzymes essential for glutathione biosynthesis. The compound's structural similarity to glutamine allows it to bind to active sites of key enzymes, preventing normal substrate utilization and disrupting the enzymatic cascade leading to glutathione formation. Gamma-glutamylcysteine synthetase, the rate-limiting enzyme in glutathione synthesis, becomes compromised when 6-Diazo-5-Oxo-L-Norleucine competes with glutamine for binding sites. This competitive inhibition reduces the availability of gamma-glutamylcysteine, the immediate precursor to glutathione, effectively limiting overall glutathione production capacity. The compound's high affinity for glutamine-binding sites ensures sustained inhibition even at relatively low concentrations. Research demonstrates that cells treated with this compound show dramatic reductions in glutathione levels within hours of exposure, indicating rapid and efficient disruption of normal biosynthetic pathways.
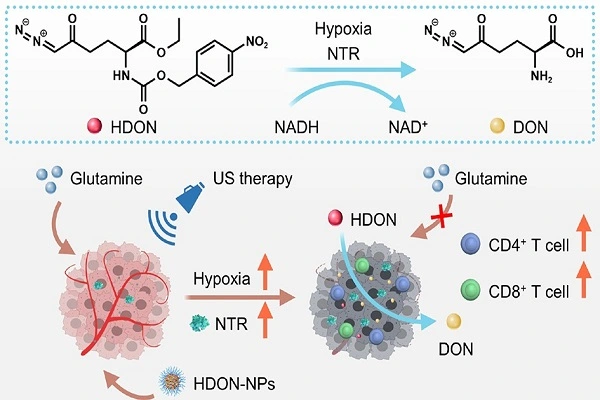
Enzyme Active Site Binding
The molecular structure of 6-Diazo-5-Oxo-L-Norleucine enables specific binding interactions with glutamine-utilizing enzymes through hydrogen bonding and electrostatic interactions that mimic natural substrate binding. The diazo group provides unique chemical properties that enhance binding affinity while preventing normal enzymatic catalysis, creating irreversible inhibition in many cases. X-ray crystallography studies reveal that this compound occupies glutamine binding pockets with high specificity, forming stable enzyme-inhibitor complexes that resist dissociation. The compound's ability to form covalent bonds with active site residues contributes to its potent and long-lasting inhibitory effects on glutathione synthesis pathways. Multiple enzymes involved in glutamine metabolism become simultaneously affected, creating a cascade of metabolic disruptions that extend beyond simple competitive inhibition. This comprehensive enzymatic disruption explains why 6-Diazo-5-Oxo-L-Norleucine produces such profound effects on cellular glutathione pools with relatively modest dosing requirements.
Metabolic Pathway Disruption
6-Diazo-5-Oxo-L-Norleucine creates comprehensive disruption of glutamine-dependent metabolic networks that support glutathione production and maintenance. The compound interferes with glutaminolysis, the process by which cells convert glutamine to glutamate, thereby reducing the availability of glutamate for glutathione synthesis. Mitochondrial glutaminase activity becomes severely compromised, limiting the production of glutamate and alpha-ketoglutarate that serve as carbon skeletons for amino acid synthesis. The disruption extends to transamination reactions that normally produce cysteine and glycine, the other two amino acids required for glutathione formation. Cells exposed to this compound experience reduced ATP production from glutamine oxidation, limiting the energy available for energy-dependent glutathione synthesis reactions. The compound also affects glutathione recycling pathways by disrupting NADPH production from glutamine-dependent processes, further compromising cellular antioxidant capacity and creating metabolic stress conditions that researchers can exploit for experimental purposes.
Cellular Response Mechanisms to Glutathione Depletion
Oxidative Stress Pathway Activation
Cellular exposure to 6-Diazo-5-Oxo-L-Norleucine triggers complex oxidative stress responses as glutathione depletion compromises normal antioxidant defense systems. Reduced glutathione levels lead to accumulation of reactive oxygen species, activating stress-responsive transcription factors such as Nrf2 and NF-κB that attempt to restore cellular redox balance. The compound's effect on glutathione production creates conditions similar to those observed in various pathological states, making it valuable for studying disease mechanisms and potential therapeutic interventions. Cells respond by upregulating alternative antioxidant systems, including catalase, superoxide dismutase, and peroxiredoxins, though these compensatory mechanisms often prove insufficient to fully restore normal redox homeostasis. Heat shock proteins and other molecular chaperones become activated in response to protein oxidation resulting from glutathione depletion. The cellular stress response provides researchers with opportunities to identify novel therapeutic targets and understand how cells adapt to oxidative challenges in cancer, neurodegeneration, and aging processes.
Apoptotic Signaling Cascades
6-Diazo-5-Oxo-L-Norleucine-induced glutathione depletion activates multiple apoptotic pathways through oxidative damage to cellular components and disruption of normal survival signaling. Mitochondrial membrane integrity becomes compromised as glutathione depletion reduces the cell's ability to maintain proper redox balance, leading to cytochrome c release and caspase activation. The compound's effects on glutathione levels sensitize cells to apoptotic stimuli, making it particularly useful for cancer research applications where selective tumor cell killing is desired. p53 tumor suppressor pathways become activated in response to oxidative DNA damage caused by glutathione insufficiency, triggering cell cycle arrest and programmed cell death in genetically intact cells. Cancer cells with defective p53 responses may show altered sensitivity patterns, providing insights into therapeutic selectivity mechanisms. The differential apoptotic responses observed with this compound help researchers understand how glutathione depletion contributes to cell death in various disease contexts and identify potential combination therapy approaches.
Metabolic Reprogramming Responses
Cells treated with 6-Diazo-5-Oxo-L-Norleucine undergo extensive metabolic reprogramming as they attempt to compensate for glutathione depletion and maintain essential cellular functions. Alternative pathways for cysteine acquisition become upregulated, including increased cystine uptake through system xc- transporters and enhanced methionine cycle activity for cysteine synthesis. Glucose metabolism shifts toward the pentose phosphate pathway to generate NADPH required for glutathione recycling and antioxidant defense systems. The compound's impact on glutamine metabolism forces cells to rely more heavily on other amino acids for energy production and biosynthetic processes. Autophagy pathways become activated as cells attempt to recycle damaged proteins and organelles resulting from oxidative stress. These metabolic adaptations provide valuable insights into cellular flexibility and identify potential vulnerabilities that could be exploited for therapeutic purposes. Understanding how different cell types respond to glutathione depletion helps researchers develop more effective treatment strategies for diseases characterized by oxidative stress and metabolic dysfunction.
Research Applications and Therapeutic Implications
Cancer Cell Vulnerability Studies
6-Diazo-5-Oxo-L-Norleucine serves as an invaluable tool for investigating cancer cell vulnerabilities related to glutathione metabolism and oxidative stress resistance. Many cancer cells exhibit increased dependence on glutamine metabolism for growth and survival, making them particularly susceptible to glutathione depletion strategies. The compound enables researchers to identify tumor types that rely heavily on glutathione-mediated antioxidant defenses, revealing potential therapeutic windows for oxidative stress-based treatments. Combination studies with other anticancer agents demonstrate synergistic effects when glutathione levels are reduced, enhancing the efficacy of chemotherapy and radiation therapy approaches. Drug resistance mechanisms often involve enhanced glutathione production, and this compound helps researchers understand these protective mechanisms and develop strategies to overcome them. The selective toxicity observed in certain cancer cell lines provides insights into metabolic differences between normal and transformed cells. These findings contribute to the development of glutamine-targeting therapeutic approaches that could improve cancer treatment outcomes while minimizing toxicity to healthy tissues.
Metabolic Disease Research Applications
Researchers utilize 6-Diazo-5-Oxo-L-Norleucine to investigate metabolic diseases characterized by altered glutathione homeostasis and oxidative stress, including diabetes, fatty liver disease, and metabolic syndrome. The compound's ability to modulate glutathione levels provides insights into how antioxidant deficiency contributes to disease progression and complications. Studies examining insulin resistance and glucose metabolism benefit from controlled glutathione depletion to understand the role of oxidative stress in metabolic dysfunction. Liver research applications focus on understanding how glutathione depletion affects detoxification processes and contributes to hepatotoxicity and fibrosis development. The compound enables investigation of age-related metabolic changes, as glutathione levels naturally decline with aging and contribute to increased disease susceptibility. Cardiovascular research applications examine how glutathione depletion affects endothelial function and contributes to atherosclerosis and hypertension development. These diverse research applications demonstrate the compound's versatility in advancing our understanding of metabolic health and disease, potentially leading to new therapeutic approaches for managing metabolic disorders.
Neuroscience and Neurodegeneration Studies
6-Diazo-5-Oxo-L-Norleucine provides neuroscience researchers with a powerful tool for studying glutathione's role in brain function and neurodegenerative disease processes. The brain's high oxygen consumption and limited antioxidant capacity make it particularly vulnerable to glutathione depletion, creating relevant disease models for conditions like Parkinson's, Alzheimer's, and Huntington's diseases. The compound enables investigation of how glutathione deficiency contributes to neuroinflammation and glial cell activation observed in neurodegenerative conditions. Studies of synaptic function and neurotransmitter metabolism reveal how oxidative stress affects neuronal communication and cognitive function. The compound's effects on glutamate metabolism are particularly relevant for understanding excitotoxicity and its role in stroke and traumatic brain injury. Research into developmental neuroscience benefits from controlled glutathione modulation to understand how oxidative stress affects brain development and maturation processes. These applications contribute to identifying potential neuroprotective strategies and therapeutic targets for treating neurodegenerative diseases, highlighting the critical importance of maintaining proper glutathione balance for optimal brain health and function.
Conclusion
6-Diazo-5-Oxo-L-Norleucine emerges as a crucial research tool for understanding glutathione metabolism and its impact on cellular function across diverse biological systems. Its unique mechanism of action provides researchers with unprecedented opportunities to investigate oxidative stress responses, metabolic adaptations, and therapeutic vulnerabilities in various disease contexts.
Xi'an Faithful BioTech Co., Ltd. proudly offers premium-grade 6-Diazo-5-Oxo-L-Norleucine that meets the exacting standards required for cutting-edge research applications. Our commitment to quality and innovation ensures that researchers worldwide have access to reliable, high-purity compounds essential for advancing scientific knowledge. Discover how our superior 6-Diazo-5-Oxo-L-Norleucine can accelerate your research breakthroughs and contribute to meaningful scientific discoveries. Contact our expert team today at allen@faithfulbio.com to discuss your specific research requirements and experience the difference that exceptional quality makes.
References
1. Griffith, O.W. & Meister, A. (1979). Potent and Specific Inhibition of Glutathione Synthesis by Buthionine Sulfoximine. Journal of Biological Chemistry, 254(16), 7558-7560.
2. Huang, C.S. & Anderson, M.E. (1991). Glutathione: An Overview of Biosynthesis and Modulation. Chemico-Biological Interactions, 111-112, 145-166.
3. Lash, L.H. (2006). Mitochondrial Glutathione Transport: Physiological, Pathological and Toxicological Implications. Chemico-Biological Interactions, 163(1-2), 54-67.
4. Lu, S.C. (2009). Regulation of Glutathione Synthesis. Molecular Aspects of Medicine, 30(1-2), 42-59.
5. Meister, A. & Anderson, M.E. (1983). Glutathione. Annual Review of Biochemistry, 52, 711-760.
6. Wu, G., Fang, Y.Z., Yang, S., Lupton, J.R. & Turner, N.D. (2004). Glutathione Metabolism and Its Implications for Health. Journal of Nutrition, 134(3), 489-492.











