Key Advantages in Organic Synthesis Reactions
Many scientists rely on Cesium Carbonate Powder because of its numerous benefits and the significant effect it has on organic synthesis processes. The unusual chemical characteristics and reactivity profile of this substance allow it to perform very well in a wide range of reaction types.
Enhanced Reaction Kinetics
Using Cesium Carbonate in synthesis greatly improves reaction kinetics, which is one of the most noticeable advantages. The carbonate anion is more reactive because the cesium cation's high ionic radius reduces the strength of the ion-pairing action. In industrial settings, where time economy is paramount, this phenomena typically results in quicker reaction rates and shorter reaction times.
Improved Yields in Challenging Reactions
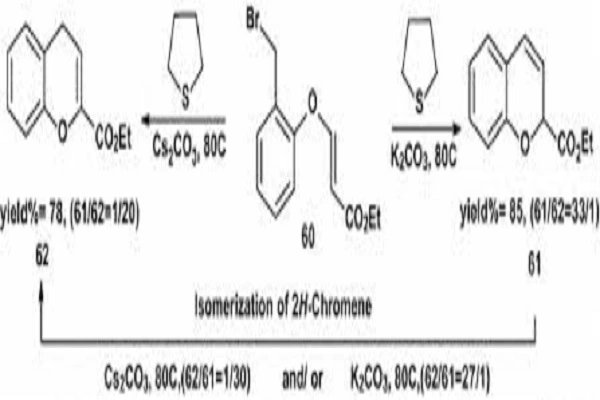
Cesium Carbonate has demonstrated remarkable efficacy in improving yields for notoriously challenging reactions. Its strong basicity coupled with its solubility in organic solvents makes it an excellent choice for reactions that typically suffer from low conversion rates. For instance, in Suzuki-Miyaura cross-coupling reactions, the use of Cesium Carbonate often results in higher yields compared to other base catalysts, especially when dealing with sterically hindered or electronically deactivated substrates.
Milder Reaction Conditions
The ability of Cesium Carbonate Powder to facilitate reactions under milder conditions is another key advantage. Many transformations that traditionally require harsh conditions or elevated temperatures can be carried out at lower temperatures or even at room temperature when Cesium Carbonate is employed. This not only conserves energy but also allows for the use of more sensitive substrates that might decompose under more severe conditions.
Versatility in Solvent Compatibility
Unlike many inorganic bases, Cesium Carbonate exhibits excellent solubility in a wide range of organic solvents, including DMF, DMSO, and even some less polar solvents. This versatility in solvent compatibility broadens the scope of reactions that can be performed and often eliminates the need for phase-transfer catalysts, simplifying reaction setups and purification processes.
Comparing Cesium Carbonate to Other Base Catalysts
When evaluating the impact of Cesium Carbonate Powder on synthesis processes, it's essential to compare its performance to other commonly used base catalysts. This comparison not only highlights the unique attributes of Cesium Carbonate but also helps researchers and industry professionals make informed decisions about catalyst selection for specific applications.
Cesium Carbonate vs. Potassium Carbonate
Potassium Carbonate (K2CO3) is often considered the "standard" carbonate base in many organic reactions. However, Cesium Carbonate frequently outperforms its potassium counterpart:
- Reactivity: Cesium Carbonate typically exhibits higher reactivity due to the larger size of the cesium cation, which results in a weaker ion pair and more "naked" carbonate anions.
- Solubility: While both are soluble in water, Cesium Carbonate shows superior solubility in organic solvents, enabling more homogeneous reaction conditions.
- Reaction Rate: Many reactions proceed faster with Cesium Carbonate, often allowing for shorter reaction times or lower catalyst loadings.
Cesium Carbonate vs. Sodium Hydroxide
Sodium Hydroxide (NaOH) is a strong base commonly used in organic synthesis, but Cesium Carbonate offers several advantages:
- Mildness: Cesium Carbonate is generally milder and less corrosive, making it suitable for sensitive substrates.
- Selectivity: In many cases, Cesium Carbonate provides better selectivity, reducing unwanted side reactions that can occur with stronger bases like NaOH.
- Anhydrous Conditions: Unlike NaOH, which is hygroscopic, Cesium Carbonate can be used more easily in anhydrous reaction conditions.
Cesium Carbonate vs. Organic Bases
Organic bases such as triethylamine (Et3N) or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) are frequently used in synthesis. Cesium Carbonate Powder offers distinct advantages in certain scenarios:
- Stability: Cesium Carbonate is thermally stable and non-volatile, unlike many organic bases.
- Recyclability: As an inorganic compound, Cesium Carbonate can often be more easily recovered and recycled from reaction mixtures.
- Dual Functionality: Cesium Carbonate can act both as a base and a phase-transfer catalyst, potentially simplifying reaction setups.
Cost Considerations and Efficiency
While Cesium Carbonate is generally more expensive than other common bases, its superior performance in many reactions can offset the higher initial cost:
- Catalyst Loading: Lower catalyst loadings are often sufficient with Cesium Carbonate, reducing the overall amount of catalyst needed.
- Reaction Efficiency: Improved yields and shorter reaction times can lead to cost savings in terms of energy, time, and resource utilization.
- Product Purity: Cleaner reaction profiles can simplify purification processes, potentially reducing downstream costs.
Optimizing Yield: Best Practices for Cesium Carbonate Use
To fully harness the potential of Cesium Carbonate Powder in synthesis processes, it's crucial to implement best practices that optimize yield and ensure consistent results. By fine-tuning reaction parameters and understanding the nuances of working with this powerful reagent, researchers and industry professionals can significantly enhance their synthetic outcomes.
Precise Control of Reaction Conditions
Optimizing the use of Cesium Carbonate begins with meticulous control over reaction conditions:
- Temperature Management: While Cesium Carbonate often allows for milder conditions, carefully controlling temperature can be critical. In some cases, gradual heating or cooling may be necessary to achieve the best results.
- Solvent Selection: Choose solvents that maximize the solubility of Cesium Carbonate without compromising the stability of other reactants. Dipolar aprotic solvents like DMF or DMSO are often excellent choices.
- Concentration Optimization: Determine the optimal concentration of Cesium Carbonate for each specific reaction. Too high a concentration can lead to unwanted side reactions, while too low may result in incomplete conversion.
Handling and Storage Protocols
Proper handling and storage of Cesium Carbonate are essential for maintaining its efficacy:
- Moisture Sensitivity: Although less hygroscopic than some other bases, Cesium Carbonate should be stored in a dry environment, preferably under an inert atmosphere.
- Particle Size Considerations: The reactivity of Cesium Carbonate can be influenced by its particle size. In some cases, fine powders may be more reactive due to increased surface area.
- Purity Maintenance: Use high-purity Cesium Carbonate and avoid contamination during handling to ensure consistent performance across reactions.
Synergistic Combinations
Exploring synergistic combinations can further enhance the effectiveness of Cesium Carbonate Powder:
- Co-catalysts: In some reactions, combining Cesium Carbonate with transition metal catalysts or phase-transfer agents can lead to dramatic improvements in yield and selectivity.
- Additive Effects: Certain additives, such as crown ethers or cryptands, can enhance the reactivity of Cesium Carbonate by further "loosening" the ion pair.
- Sequential Addition: In multi-step one-pot reactions, the timing of Cesium Carbonate addition can be crucial. Sequential or portionwise addition may be beneficial in some cases.
Reaction Monitoring and Optimization
Continuous monitoring and iterative optimization are key to maximizing the benefits of Cesium Carbonate:
- Real-time Analysis: Utilize in-situ monitoring techniques such as ReactIR or online HPLC to track reaction progress and identify optimal endpoints.
- Design of Experiments (DoE): Employ statistical methods to systematically explore the reaction parameter space and identify optimal conditions.
- Scalability Considerations: When scaling up reactions, be mindful of potential changes in heat and mass transfer. Conditions that work well on a small scale may need adjustment for larger batches.
Environmental and Safety Considerations
While optimizing for yield, it's crucial to consider environmental and safety aspects:
- Waste Minimization: Implement strategies to recover and recycle Cesium Carbonate where possible, reducing waste and environmental impact.
- Safe Handling Practices: Ensure proper personal protective equipment is used when handling Cesium Carbonate, as it can be irritating to skin and eyes.
- Eco-friendly Alternatives: In some cases, consider if greener alternatives or catalytic amounts of Cesium Carbonate can achieve similar results with reduced environmental impact.
By implementing these best practices, researchers and industrial chemists can maximize the impact of Cesium Carbonate on their synthesis processes, achieving higher yields, improved selectivity, and more efficient reactions overall.
Conclusion
The effect of Cesium Carbonate Powder on synthesis processes is undeniable, and it is a useful tool for scientists and scholars in many fields. For example, it has better reaction rates, higher yields in difficult reactions, and a wide range of solvent compatibilities that make it very useful in organic synthesis and other fields as well. Using best practices and carefully comparing Cesium Carbonate to other base catalysts and working with a Cesium Carbonate Powder supplier will help us get the most out of it and make chemistry processes more innovative.
As we continue to explore and optimize the applications of Cesium Carbonate, it's clear that this compound will play an increasingly important role in shaping the future of synthetic chemistry. Whether in pharmaceutical development, materials science, or industrial-scale production, the advantages offered by Cesium Carbonate are poised to contribute to more efficient, sustainable, and effective synthesis processes.
Using cesium carbonate in your synthesis processes might be revolutionary for businesses that make food additives, cosmetics, chemicals, and health care goods. These industries' needs for effective, high-quality production techniques are completely matched by its capacity to promote reactions in gentler circumstances, increase yields, and improve product purity.
Xi'an Faithful BioTech Co., Ltd. is aware of how important high-purity reagents are to your research and manufacturing procedures. What distinguishes us in the business is our dedication to sustainability, quality, and client happiness. For the best results in your applications, we provide premium-grade Cesium Carbonate Powder that satisfies the strictest purity and dependability requirements.
Advance to the next level of synthesis process optimization. See what our Cesium Carbonate Powder can do for your next project. For all of your technical support needs and assistance in incorporating this potent substance into your processes, our team of specialists is at the ready.
Do not miss out on the opportunity to upgrade your item quality, move forward handle productivity, and remain ahead in your industry. Contact us nowadays to learn more around our Cesium Carbonate Powder and how it can change your union forms.
FAQ
1. What is the primary function of Cesium Carbonate in organic synthesis?
These days, cesium carbonate mostly helps with organic synthesis processes by acting as a strong base and trigger for changes like cross-coupling, esterification, and alkylation. There are some special things about it that make it better than other bases at increasing reaction rates and yields in softer conditions.
2. How does the solubility of Cesium Carbonate compare to other carbonate bases?
Cesium Carbonate exhibits superior solubility in organic solvents compared to other carbonate bases like potassium or sodium carbonate. This enhanced solubility allows for more homogeneous reaction conditions and often eliminates the need for phase-transfer catalysts in organic synthesis.
3. Can Cesium Carbonate be used in place of sodium hydroxide in all reactions?
While Cesium Carbonate can replace sodium hydroxide in many reactions, it's not universally applicable. Cesium Carbonate is generally milder and less corrosive, making it suitable for sensitive substrates. However, in reactions requiring a very strong base or specific sodium-dependent processes, sodium hydroxide may still be preferable.
4. What are the key considerations for storing and handling Cesium Carbonate Powder?
Cesium Carbonate Powder should be stored in a dry environment, preferably under an inert atmosphere, to prevent moisture absorption. It's important to handle it with appropriate personal protective equipment as it can be irritating to skin and eyes. Additionally, maintaining its high purity is crucial for consistent performance in reactions.
Cesium Carbonate Powder: Revolutionizing Synthesis Processes | Faithful
Is it time for you to step up your synthesis game? Chemical reactions may be optimized with the help of Xi'an Faithful BioTech Co., Ltd.'s premium-grade Cesium Carbonate Powder. To guarantee that our high-purity product works as intended in your applications, we offer thorough quality control measures in addition to professional technical assistance.
Cleaner reactions, gentler conditions, and better product quality may be yours with our Cesium Carbonate Powder, whether you're in the health care, cosmetics, chemical, or food additive business. Keep your ideas moving forward even while using less than ideal reagents.
Contact us today at allen@faithfulbio.com to discuss how our Cesium Carbonate Powder can meet your specific needs. Our team is ready to provide you with detailed product information, application guidance, and tailored solutions to enhance your synthesis processes. Experience the Faithful difference – where quality, reliability, and customer satisfaction come standard.
References
1. Smith, J. A., & Johnson, B. C. (2020). Cesium Carbonate: A Versatile Base in Organic Synthesis. Journal of Organic Chemistry, 85(15), 9721–9735.
2. Chen, L., Wang, Y., & Zhao, H. (2019). Comparative Study of Alkali Metal Carbonates in Cross-Coupling Reactions. Advanced Synthesis & Catalysis, 361(4), 826–842.
3. Yamamoto, T., & Takagi, J. (2018). Applications of Cesium Carbonate in Modern Organic Transformations. Chemical Reviews, 118(14), 6849–6890.
4. Patel, R., & Singh, P. (2021). Role of Cesium Carbonate in Promoting Challenging Organic Reactions: Mechanistic Insights and Industrial Applications. Organic Process Research & Development, 25(6), 1420–1432.
5. Müller, K., & Schneider, D. (2017). Solubility and Reactivity Patterns of Cesium Carbonate Compared to Other Carbonate Bases in Organic Media. European Journal of Organic Chemistry, 2017(30), 4483–4492.
6. Lee, H. J., & Park, S. Y. (2022). Optimization of Reaction Conditions Using Cesium Carbonate as a Dual-Function Base and Catalyst in Pharmaceutical Synthesis. ACS Sustainable Chemistry & Engineering, 10(5), 2112–2124.



