Remdesivir powder :How to refine the 'secret weapon' of the antiviral campaign?
Remdesivir powder, an antiviral drug, was originally designed for Ebola virus, and was used urgently to treat COVID-19 infection during the COVID-19 epidemic. Clinical trials have shown that it can moderately shorten the recovery time of some hospitalized patients, but its efficacy is limited for critically ill patients and needs to be combined with other treatment methods.
Development history: "cross-border heroes" from Ebola to COVID-19
The birth of remdesivir can be called a "cross-border legend" of modern drug research and development. In 2015, when it first appeared in the public eye, the target was the Ebola virus. At that time, the African continent was experiencing the Ebola epidemic. Scientists found that remdesivir showed inhibitory activity against a variety of RNA viruses in vitro experiments, including Nipah virus and Middle East Respiratory Syndrome (MERS) coronavirus.

In 2016, the Phase I clinical trial of Ebola virus was completed, but it was not advanced due to the easing of the epidemic.
January 2020: After the outbreak of the COVID-19, Chinese scientists published a paper in The Lancet, proposing for the first time that remdesivir might be effective against COVID-19.
October 2020: the US FDA approved it for the treatment of COVID-19 inpatients, becoming the first approved anti COVID-19 drug in the world.
Nature and Shape: The "Molecular Code" Behind White Crystals
The physical properties of remdesivir powder can be called the model of "laboratory aesthetics": white to almost white crystalline powder, molecular formula C27H35N6O8P, molecular weight 602.56 g/mol. Its solubility is excellent (soluble in water and ethanol), but its stability is temperature sensitive - even after being stored at 40 ℃ for 3 months, the purity can still remain above 98%.
The chemical structure of remdesivir contains a "tricyclic skeleton", inspired by the transformation of the natural product adenosine. At the same time, in the X-ray diffraction pattern, its crystal presents a perfect hexagonal system, resembling a "molecular snowflake".
Main use: not only COVID-19's "broad-spectrum soldiers"
Remdesivir's "ambition" is far beyond COVID-19. At present, its approved indications include:
1. COVID-19 infection: shorten the recovery time of hospitalized patients.
A total of 1062 patients underwent randomization (with 541 assigned to remdesivir and 521 to placebo). Those who received remdesivir had a median recovery time of 10 days (95% confidence interval [CI], 9 to 11), as compared with 15 days (95% CI, 13 to 18) among those who received placebo (rate ratio for recovery, 1.29; 95% CI, 1.12 to 1.49; P<0.001, by a log-rank test). In an analysis that used a proportional-odds model with an eight-category ordinal scale, the patients who received remdesivir were found to be more likely than those who received placebo to have clinical improvement at day 15 (odds ratio, 1.5; 95% CI, 1.2 to 1.9, after adjustment for actual disease severity). The Kaplan–Meier estimates of mortality were 6.7% with remdesivir and 11.9% with placebo by day 15 and 11.4% with remdesivir and 15.2% with placebo by day 29 (hazard ratio, 0.73; 95% CI, 0.52 to 1.03). Serious adverse events were reported in 131 of the 532 patients who received remdesivir (24.6%) and in 163 of the 516 patients who received placebo (31.6%)
so data show that remdesivir powder was superior to placebo in shortening the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection.

2. Ebola virus: Although not ultimately approved, phase III trials have shown a 30% reduction in mortality rates
Patients in the remdesivir group received a loading dose on day 1 (200 mg in adults, and adjusted for body weight in pediatric patients), followed by a daily maintenance dose (100 mg in adults) starting on day 2 and continuing for 9 to 13 days, depending on viral load.The mean baseline creatinine and aspartate aminotransferase values were higher in the ZMapp and remdesivir groups than in the other two groups, an analysis of mortality showed that there was a clear separation between the MAb114 group and the ZMapp and remdesivir groups.the difference between the remdesivir and ZMapp groups was 3.4 percentage points.
3. Potential expansion areas: monkeypox, influenza, respiratory syncytial virus (RSV), etc
In vitro experiments showed that rheosevir could inhibit the replication of monkeypox virus, which might work by interfering with viral RNA synthesis.
During the monkeypox outbreak in 2022, some clinical doctors attempted to use it for critically ill patients, but lacked large-scale clinical trial data support.
In vitro experiments showed that remdesivir had a certain inhibitory effect on influenza A and B viruses, possibly by blocking viral RNA replication. In animal models, early administration can alleviate lung damage, but the effect is weaker than Oseltamivir
One study showed that Redsivir can inhibit RSV replication. In animal models, Redsivir can reduce the viral load and reduce lung inflammation. A clinical trial (NCT05123313) will be conducted in 2023 to evaluate its efficacy in hospitalized RSV patients, and the results are yet to be announced.
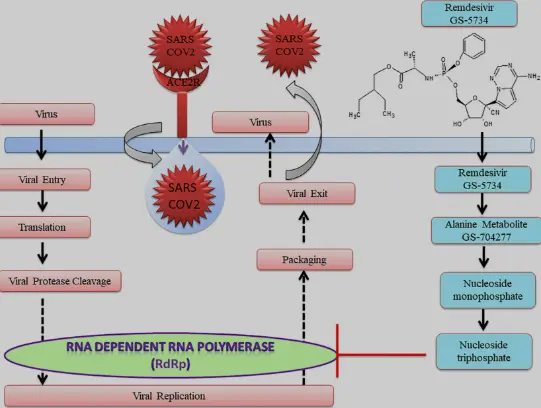
Mechanism of action: "Agents" infiltrating virus factories
As an adenosine nucleoside triphosphate analog (GS-443902),the active metabolite of remdesivir interferes with the action of viral RNA-dependent RNA polymerase and evades proofreading by viral exoribonuclease (ExoN), causing a decrease in viral RNA production. In some viruses, such as the respiratory syncytial virus, it causes the RNA-dependent RNA polymerases to pause, but its predominant effect (as in Ebola) is to induce an irreversible chain termination. Unlike with many other chain terminators, this is not mediated by preventing addition of the immediately subsequent nucleotide, but is instead delayed, occurring after five additional bases have been added to the growing RNA chain. For the RNA-dependent RNA polymerases of MERS-CoV, SARS-CoV-1, and SARS-CoV-2, arrest of RNA synthesis occurs after incorporation of three additional nucleotides. Hence, remdesivir is classified as a direct-acting antiviral agent that works as a delayed chain terminator.
In short,as a nucleoside analog prodrug, remdesivir's killing trick is "camouflage":
1. Infiltration: After entering the host cell, it is converted into the active triphosphate form (GS-441524).
2. Destruction: Embedding virus RNA strands, inducing "error catastrophe" and forcing virus replication to terminate.
3. Broad spectrum: Effective against viruses that rely on RNA polymerase, such as coronaviruses and filamentous viruses.
Related research: The 'data storm' from laboratory to clinical practice
Milestone research
ACTT-1 test (2020): among 397 patients, the recovery time of remdesivir powder group was shortened by 5 days (p=0.0045).
ACTT-3 trial (2021): Combined treatment with Baricitinib further reduced mortality by 11%.
Controversy and Breakthrough
In 2022, the journal Nature pointed out that the efficacy of remdesivir on Delta variants declined, but the updated data in 2023 showed that it remained active on Omicron BA. 5 (IC50=0.15 μ M).
From laboratory synthesis to industrial production
Remdesivir can be synthesized in multiple steps from ribose derivatives.
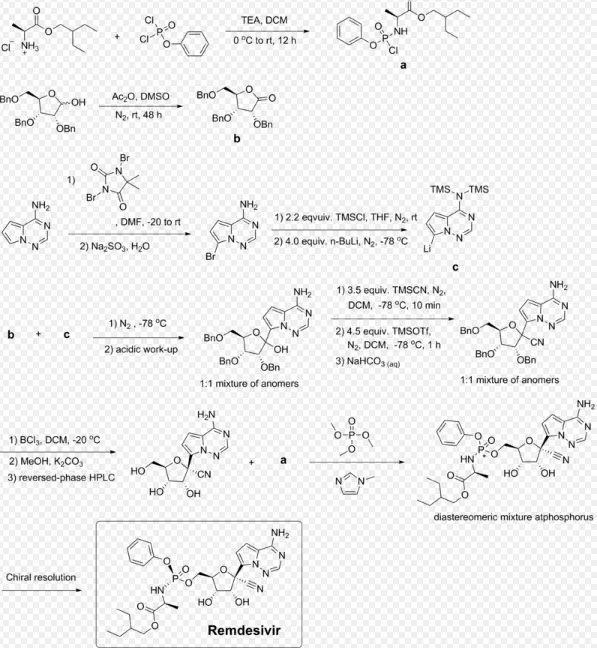
Remdesivir requires "70 raw materials, reagents, and catalysts" to make, and approximately "25 chemical steps."Some of the ingredients are extremely dangerous to humans, especially trimethylsilyl cyanide.The original end-to-end manufacturing process required 9 to 12 months to go from raw materials at contract manufacturers to finished product, but after restarting production in January 2020, factory was able to find ways to reduce the production time to six months.
Future Outlook: Evolution from COVID-19 to "Super Antibiotics"
Three major directions of remdesivir's recent research
1. Expansion of indications: Phase II/III trials of remdesivir against monkeypox have been started,
2. Long acting formulation: Develop subcutaneous injection form, maintain efficacy for 2 weeks with a single dose.
3. Combination therapy: Used in combination with neutralizing antibodies and immune modulators to address viral resistance.
Anthony Fauc, director of the National Institute of Allergy and Infectious Diseases (NIAID), predicted that "in the next five years, remdesivir may become the" first-line drug reserve "for respiratory virus infection."
Conclusion
The story of remdesivir powder is not only a subtle adjustment of the molecular structure in the laboratory, but also a microcosm of human scientific wisdom and cooperation spirit under the threat of viruses. From Ebola to COVID-19, every structural transformation promotes the boundary of life science, and every application dispute reminds us that anti-virus is not only a technical problem, but also a test of the global governance system.
Xi'an Faithful BioTech Co., Ltd. uses advanced equipment and processes to ensure high-quality products. We produce high-quality remdesivir powder, that meet international drug standards. Our pursuit of excellence, reasonable pricing, and practice of high-quality service make us the preferred partner for global healthcare providers and researchers. If you need to conduct scientific research or production of remdesivir , please contact our technical team through the following methods:sales12@faithfulbio.com.
Reference
1 John H. Beigel, M.DKay M. Tomashek and M.D., M.P.H., Lori E. Dodd. Remdesivir for the Treatment of Covid-19 — Final Report. May 22, 2020 N Engl J Med 2020;383:1813-1826
2 Cho A, Saunders OL, Butler T, Zhang L, Xu J, Vela JE, et al. (April 2012). "Synthesis and antiviral activity of a series of 1'-substituted 4-aza-7,9-dideazaadenosine C-nucleosides". Bioorganic & Medicinal Chemistry Letters. 22 (8): 2705–2707.
3 Ferner RE, Aronson JK (April 2020). "Remdesivir in covid-19". BMJ. 369 m1610. doi:10.1136/bmj.m1610. PMID 32321732. Archived from the original on 8 March 2021. Retrieved 30 April 2020.
4 Tchesnokov EP, Feng JY, Porter DP, Götte M (April 2019). "Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir". Viruses. 11 (4): 326.
5 Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M (April 2020). "The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus". The Journal of Biological Chemistry. 295 (15): 4773–4779.



