Why is Zonisamide API Powder more and more popular in the field of neurodrugs?
Who's Zonisamide API Powder ? -From "antiepileptic drugs" to "nerve generalists"
Its chemical structure is quite special, belonging to benzoxazole derivatives. Simply put, Zonisamide API Powder has a special ring structure in its molecular structure, which allows it to act on nerve cells in the brain in many ways.
1. Mechanism of action: More than one "weapon"
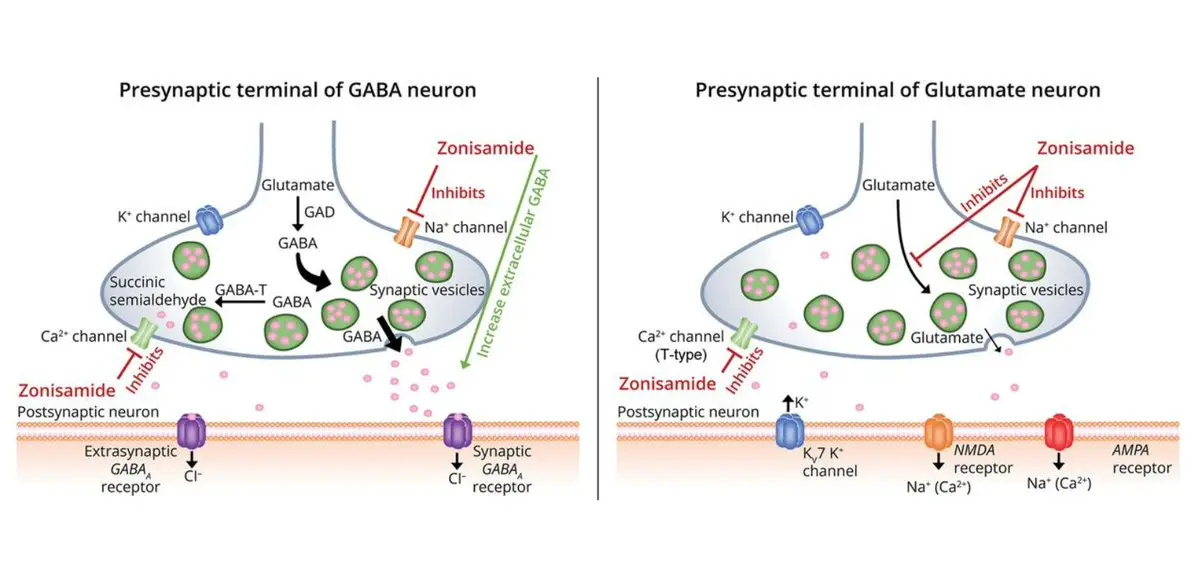
Traditional antiepileptic products are often only aimed at a certain "switch" of nerve cells, while Zonisamide is like a "generalist" with several "weapons":
Weapon 1: Turn off the "random discharge" switch (blocking individual channels). The reason why nerve cells are extremely excited and lead to seizures is because the "channel" on the cell membrane is opened, allowing a large number of charged particles to flood in. It can block these channels like a plug and reduce the abnormal discharge of nerve cells.
Weapon 2: Suppress the "low frequency oscillator" (blocking the T-type calcium channel). Nerve cells in some areas of the brain (such as thalamus) will discharge rhythmically like a pendulum. If this rhythm is out of control, it will lead to absences and other types of epilepsy. Zonisamide can inhibit this "T-type calcium channel" and stop the pendulum.
Weapon 3: Adjust the "cellular environment" (slightly inhibit carbonic anhydrase). Carbonic anhydrase is an enzyme that regulates acid-base balance in cells. It can slightly inhibit it and change the acid-base environment in cells, thus indirectly reducing the excitability of nerve cells.
Weapon 4: Enhance the "sedation signal" (which may enhance GABA). GABA is the main "sedative" in the brain, which can "calm down" nerve cells. Some studies believe that Zonisamide can enhance the effect of GABA. Although this effect is relatively weak, it also contributes a lot.
Because there are so many "weapons", Zonisamide can not only treat many types of epilepsy, but also be used for other nervous system diseases, such as motor symptoms of Parkinson's disease, prevention of migraine, and even some neuropathic pain. This "multi-target" feature makes it more advantageous than some traditional products.
2. Zonisamide API Powder: the "source of living water" of drugs
Zonisamide API Powder is the bulk drug powder of Zonisamide. Its quality directly determines the safety and effectiveness of the final drug. If the raw materials are not pure enough, or contain harmful impurities, the produced drugs may have poor effects and even cause harm to patients. Therefore, we strictly manage the production and quality control of Zonisamide .
How was Zonisamide "born"?
Producing Zonisamide API Powder is a complex and delicate process, just like building a precise instrument, each step needs precise control. The whole process can be roughly divided into the following key steps:
1. Preparation and synthesis of raw materials
The production of Zonisamide needs to start from some basic chemical raw materials and build its molecular structure step by step through a series of chemical reactions. This process is like building a house with Lego bricks, and the correct "building blocks" (chemical groups) need to be connected in the right order. Among them, the most critical step is to construct its core structure-benzoisoxazole ring and connect sulfanilamide groups. These reactions need to be carried out under specific temperature, pressure and catalyst conditions to ensure the complete reaction and minimize the formation of by-products.
2. Purification and crystallization-panning out "gold" from "sand"
After the chemical reaction is completed, there are many "impurities" such as reaction by-products, unreacted raw materials, solvents, etc. in the obtained mixture besides the Zonisamide we want. At this time, purification is needed. The most commonly used method is crystallization.
The principle of crystallization is simple: Zonisamide and impurities have different solubility in different solvents and at different temperatures, so that it can slowly "grow" into neat crystals from the solution, while impurities remain in the solution and are filtered out. This process may need to be repeated several times, just like panning for gold, washing it over and over again until you get enough pure "gold"-high purity Zonisamide.
3. Drying and crushing-turning into convenient "flour"
It obtained by crystallization usually contains water or solvent, which needs to be removed by drying (such as vacuum drying). The dried crystals may be blocky, so it is necessary to crush them into uniform powder and control the particle size (particle size distribution) of the powder for the convenience of follow-up. The particles are too coarse and may not be easily mixed evenly; The particles are too fine, and it is easy to produce dust, which affects the operation and stability.
4. Quality control-the "security gate" throughout.
In the whole production process, strict quality control should be carried out at every step. Just like the assembly line in a factory, there is a "security gate" behind each process to check whether the product is qualified. Commonly used detection methods include:
HPLC (High Performance Liquid Chromatography): This is the most commonly used "magic mirror", which can accurately measure the content of Zonisamide, what impurities are there and how much impurities are there.
XRD(X-ray diffraction): used to check the "crystal form" of Zonisamide API Powder. If the crystal structure of the same drug molecule is different (just like diamond and graphite, the components are all carbon, but the structure is different and the properties are very different), its dissolution speed and efficacy may be different. We must ensure that the correct and stable crystal form is produced.
Moisture determination: ensure that the moisture content is within a safe range.
Determination of residual solvents: ensure that there is not too much residual organic solvents used in the production process, so as not to cause harm to human body.
Microbial limit inspection: make sure there are no too many bacteria or molds.
Only after passing all these "security checks" can Zonisamide API Powder be released and enter the next link-preparation production.
Zonisamide API Powder's "useful place"-application field and dosage form
Its main use is to make medicines in various dosage forms for treating the following diseases:
Its core indications: epilepsy, generalized tonic-clonic seizures, myoclonic seizures and so on.
Parkinson's disease: In some countries, Zonisamide is approved to treat the fluctuation of motor symptoms in patients with Parkinson's disease. Although this is not its main indication, it provides a new choice for patients with Parkinson's disease.
Other exploratory applications: migraine prevention: Some clinical studies show that Zonisamide may be effective in preventing migraine.
Neuropathic pain: Because of its multi-target mechanism, it has also been explored for some types of neuropathic pain.
Bipolar disorder: some studies try to use it as an auxiliary treatment of emotional stabilizer.
There are a large number of epilepsy patients in the world, and epilepsy is a chronic disease that needs long-term treatment, so the demand for anti-epilepsy products is sustained and stable. With the deepening of understanding of Zonisamide and its exploration and application in other nervous system diseases, its market demand has shown a steady growth trend.
Future prospect-the prospect of ——Zonisamide API Powder
Indication expansion: With the deepening of clinical research, it may find its application in more nervous system diseases, which will further expand its market demand.
Quality requirements are constantly improving: with the tightening of global drug supervision, the requirements for the purity, impurity control and data integrity of APIs will be higher and higher. Only suppliers who can continuously improve their quality standards can be invincible in the competition.
Globalization and regionalization of supply chain coexist: on the one hand, the supply chain of APIs is global; On the other hand, for the sake of supply chain security and cost control, some regions may tend to establish regional supply chains. The flexible response and adaptability to the needs of suppliers are also required to be improved.
Conclusion
In a word, Zonisamide, as an important raw material of nervous system drugs, its value lies not only in itself, but also in its ability to bring health and hope to millions of patients around the world. Its production, quality control and supply is a field full of challenges but also opportunities. With the progress of science and technology and the development of the industry, we have reason to believe that it will continue to play an important role in the field of nerve products and contribute to the cause of human health.
Xi'an Faithful BioTech Co., Ltd. combines cutting-edge production technology with comprehensive quality assurance to provide high-quality Zonisamide API Powder that meets international pharmaceutical standards. Our commitment to excellent, competitive prices and technical support makes us the preferred partner of global healthcare providers and researchers. Please contact our technical team in sales11@faithfulbio.com to find out how our products can improve your formula.
This is a list of the names of the core scientific research documents that I referred to and relied on in the process of writing a soft article. These documents provide solid scientific evidence for the efficacy and mechanism mentioned in this paper.
- United States Pharmacopeia (USP) – Zonisamide Monograph
- European Pharmacopoeia (Ph. Eur.) – Zonisamide Monograph
- "Zonisamide: A Review of Its Use in Epilepsy" – CNS Drugs
- "Zonisamide for Parkinson’s Disease: A Systematic Review and Meta-Analysis" – Journal of Neurology
- "Mechanisms of Action of Antiepileptic Drugs: The Role of Zonisamide" – Epilepsy Research
- "Synthesis and Characterization of Zonisamide and Its Related Substances" – Journal of Pharmaceutical Sciences
- "Crystallization and Polymorphism of Zonisamide: Impact on Dissolution and Stability" – International Journal of Pharmaceutics
- "Process Optimization for the Industrial-Scale Production of Zonisamide API" – Organic Process Research & Development
- "Development and Validation of an HPLC Method for the Determination of Zonisamide and Its Impurities" – Journal of Chromatographic Science
- "Application of X-ray Diffraction (XRD) and Thermal Analysis in the Characterization of Zonisamide Polymorphs" – Pharmaceutical Research
- "Residual Solvent Analysis in Zonisamide API by Gas Chromatography" – Journal of Pharmaceutical and Biomedical Analysis
- "Global Antiepileptic Drugs Market: Trends and Forecasts" – Grand View Research
- "API Market Outlook: Focus on Neurological Disorders" – McKinsey & Company
- "Emerging Trends in the Production of High-Purity APIs: Case Study of Zonisamide" – Chemical Weekly



